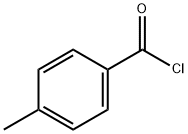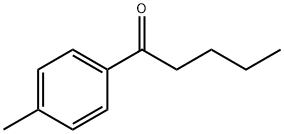
4'-Methylvalerophenone synthesis
- Product Name:4'-Methylvalerophenone
- CAS Number:1671-77-8
- Molecular formula:C12H16O
- Molecular Weight:176.25
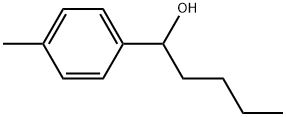
91763-35-8

1671-77-8
The general procedure for the synthesis of 4-methylpentanone from the compound (CAS: 91763-35-8) is as follows: the oxidation reaction of 1-phenyl-1-pentanol (1a) was performed as a representative operation (see Table 2, entry 1): in a reaction flask fitted with a reflux condenser and a drying tube lined with calcium chloride, BMI-PF6 (85 mg, 0.30 mmol), 1- phenyl-1-pentanol (1a, 33 mg, 0.20 mmol), Cs2CO3 (33 mg, 0.10 mmol) and trifluoromethylbenzene (0.10 mL). The reaction mixture was stirred at 105 °C for 20 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate and poured into water. The aqueous phase was extracted three times with ethyl acetate. The organic layers were combined, dried with anhydrous Na2SO4, filtered through a neutral alumina pad and concentrated under reduced pressure. Finally, purification by silica gel column chromatography using hexane/ethyl acetate (10:1, v/v) as eluent gave 1-phenyl-1-pentanone (2a, 28 mg, 0.17 mmol) in 86% yield.
Yield:1671-77-8 94 %Chromat.
Reaction Conditions:
Stage #1: 4-methyl-benzoyl chloridewith morpholine;diisobutylaluminium hydride in tetrahydrofuran;hexane at 0; for 0.166667 h;Inert atmosphere;
Stage #2: n-butyllithium in tetrahydrofuran;hexane at 0; for 0.166667 h;
Steps:
Partial alkylation of acid chlorides to corresponding ketones
General procedure: The following experimental procedure for the partial reduction of benzoyl chloride to 1-phenylpentanone is representative. A dry and argon-flushed flask, equipped with a magnetic stirring bar and a septum, was charged with morpholine (0.11 mL, 1.25 mmol) and 10 mL THF. After cooling to 0 °C, DIBALH (1.2 mL, 1.0 M in hexane, 1.2 mmol) was added dropwise and stirred for 3 h at same temperature. To the reaction mixture was slowly added benzoyl chloride (0.116 mL, 1.0 mmol) and stirred for 10 min. Then, n-BuLi (1.25 mL, 1.6 M in hexane, 2.0 mmol) was added and the mixture was stirred for 10 min again. The reaction was stopped by aqueous 1 N HCl (10 mL) and extracted with diethyl ether (2 × 10 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure. Purification of the residue by column chromatography on silica gel yielded 1-phenylpentanone (159 mg, 98%). All products in Table 3 were confirmed by comparison with NMR data reported in the literature.8
References:
Park, Jae Kyo;Shin, Won Kyu;An, Duk Keun [Tetrahedron Letters,2013,vol. 54,# 24,p. 3199 - 3203]

5030-61-5
52 suppliers
$37.00/100mg

1671-77-8
134 suppliers
$11.00/1g
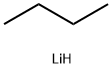
29786-93-4
0 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
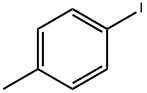
624-31-7
434 suppliers
$9.00/25g

1671-77-8
134 suppliers
$11.00/1g
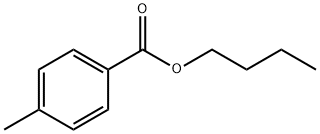
19277-56-6
3 suppliers
inquiry