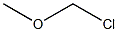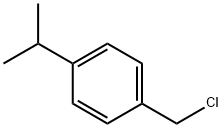
4-ISOPROPYLBENZYL CHLORIDE synthesis
- Product Name:4-ISOPROPYLBENZYL CHLORIDE
- CAS Number:2051-18-5
- Molecular formula:C10H13Cl
- Molecular Weight:168.66
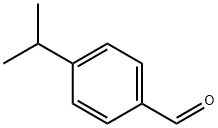
122-03-2

2051-18-5
The general procedure for the synthesis of p-isopropylbenzyl chloride from 4-isopropylbenzaldehyde was as follows: 500 ml of methanol was added slowly and dropwise over a period of 5 h to 1000 ml of THF solution containing 148 g (1.0 mol) of 4-isopropylbenzaldehyde and 37.8 g (1.0 mol) of NaBH4 at 0-5 °C, while vigorous stirring was maintained. The reaction mixture was continued to be stirred at room temperature overnight, followed by evaporation of the solvent under vacuum. The residue was acidified to pH~1 with 1200 ml of 2M HCl solution, followed by extraction of the resulting (4-isopropylphenyl) methanol with 3 x 400 ml of dichloromethane. The organic extracts were combined, dried with Na2SO4 and evaporated to dryness. The resulting residue was dissolved in 1000 ml of dichloromethane and 73 ml (1.0 mol) of thionyl chloride was added slowly and dropwise at +5 °C. The reaction solution was stirred overnight at room temperature and evaporated to dryness and the residue was dissolved in 750 ml of dichloromethane. This solution was washed with 250 ml of water and after separating the organic layer, the aqueous layer was then extracted with 2 x 150 ml of dichloromethane. All organic extracts were combined, dried over Na2SO4, filtered through a short silica gel pad (40-63 μm) and finally evaporated to dryness. The crude product was distilled under vacuum to give 142 g (84% yield) of colorless liquid with a boiling point of 107-112°C/15 mmHg.

122-03-2
362 suppliers
$6.00/25g

2051-18-5
117 suppliers
$16.39/1g
Yield: 84%
Reaction Conditions:
Stage #1:(4-isopropylbenzaldehyde) with sodium tetrahydroborate in tetrahydrofuran;methanol at 0 - 20;Inert atmosphere;Schlenk technique;Glovebox;
Stage #2: with thionyl chloride in dichloromethane at 5 - 20;Inert atmosphere;Schlenk technique;Glovebox;
Steps:
1-(Chloromethyl)-4-isopropylbenzene
500 ml of methanol was added dropwise by vigorous stirring over 5 h to a mixture of 148 g (1.0 mol) 4-isopropylbenzaldehyde and 37.8 g (1.0 mol) of NaBH4 in 1000 ml of THF at 0-5 °C. This mixture was stirred overnight at room temperature and then evaporated under vacuum. The residue was acidified with 1200 ml of 2 M HC1 to pH~l, and the formed (4-isopropylphenyl)methanol was extracted with 3 x 400 ml of dichloromethane. The combined organic extract was dried over Na2SO4 and evaporated to dryness. To the residue dissolved in 1000 ml of dichloromethane 73 ml (1.0 mol) of thionyl chloride was added dropwise at +5°C. The resulting solution was stirred at room temperature overnight, evaporated to dryness, and then the residue was dissolved in 750 ml dichloromethane. The formed solution was washed by 250 ml of water. The organic layer was separated, the aqueous layer was extracted with 2 x 150 ml of dichloromethane. The combined organic extract was dried over a2S04, passed through a short pad of silica gel 60 (40-63 μιη), and evaporated to dryness. Crude product was distilled under vacuum to give 142 g (84%) of a colorless liquid, b.p. 107-1 12 °C/15 mm Hg.
References:
BOREALIS AG;RESCONI, Luigi;VIRKKUNEN, Ville;NOUREDDINE, Ajellal;CASTRO, Pascal;IZMER, Vyatcheslav;KONONOVICH, Dmitry;VOSKOBOYNIKOV, Alexander WO2014/96282, 2014, A1 Location in patent:Page/Page column 54
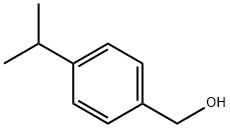
536-60-7
193 suppliers
$24.00/25mL

2051-18-5
117 suppliers
$16.39/1g
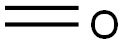
50-00-0
892 suppliers
$10.00/25g
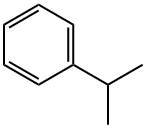
98-82-8
294 suppliers
$9.00/5ml

20034-71-3
10 suppliers
inquiry

2051-18-5
117 suppliers
$16.39/1g