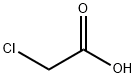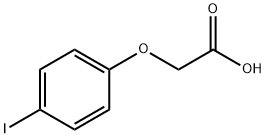
4-Iodophenoxyacetic acid synthesis
- Product Name:4-Iodophenoxyacetic acid
- CAS Number:1878-94-0
- Molecular formula:C8H7IO3
- Molecular Weight:278.04

90794-33-5

1878-94-0
The general procedure for the synthesis of p-iodophenoxyacetic acid from ethyl (4-iodophenoxy)acetate was as follows: ethyl (4-iodophenoxy)acetate (1.53 g, 5.0 mmol) was mixed with lithium hydroxide monohydrate (258 mg, 6.1 mmol) in a 1:1 mixture of tetrahydrofuran (THF) and water (12 mL) and stirred for 5 min at room temperature. Upon completion of the reaction, the reaction was quenched with 1N hydrochloric acid (HCl) solution, followed by removal of the organic solvent by distillation under reduced pressure. The crude product was extracted with ethyl acetate, washed sequentially with saturated aqueous sodium chloride (NaCl) solution and dried with anhydrous magnesium sulfate (MgSO4), and finally concentrated to give p-iodophenoxyacetic acid (1.36 g, 4.9 mmol, 98% yield) as a white solid. The product was confirmed by nuclear magnetic resonance hydrogen spectrum (1H NMR, 400 MHz, CD3OD): δ 7.48 (d, J = 8.6 Hz, 2H), 6.66 (d, J = 8.6 Hz, 2H), 4.54 (s, 2H); nuclear magnetic resonance carbon spectrum (13C NMR, 100 MHz, CD3OD): δ 172.3, 159.4, 139.4 , 118.1, 84.1, 65.8; mass spectrum (ESI) m/z 276 [M-H]-.

90794-33-5
14 suppliers
$470.00/10g

1878-94-0
210 suppliers
$10.00/1g
Yield:1878-94-0 98%
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;water at 20; for 1 h;Inert atmosphere;Schlenk technique;
Steps:
2 4.1.2 Methyl-3-[2-(4-ethynylphenoxy)acetamido]-4-hydroxybenzoate (5)
A mixture of 4 (1.53 g, 5.0 mmol), lithium hydroxide monohydrate (258 mg, 6.1 mmol) in a 1:1 solution of THF and water (12 mL) was stirred at room temperature for 5 min. The reaction was quenched by HCl solution (1 N) and the organic solvent was removed under reduced pressure. The crude products were extracted with ethyl acetate, washed with saturated aqueous NaCl solution, dried over anhydrous MgSO4, and concentrated to give product 5 as a spectroscopically pure form (1.36 g, 4.9 mmol, 98%) as a white solid: 1H NMR (400 MHz; CD3OD): δ 7.48 (d, J = 8.6 Hz, 2H), 6.66 (d, J = 8.6 Hz, 2H), 4.54 (s, 2H); 13C NMR (100 MHz; CD3OD) δ 172.3, 159.4, 139.4, 118.1, 84.1, 65.8; MS (ESI) m/z 276 [M - H]-.
References:
Nakamura, Hiroyuki;Yasui, Yuka;Ban, Hyun Seung [Journal of Organometallic Chemistry,2013,vol. 747,p. 189 - 194]
122-59-8
453 suppliers
$5.00/25g

1878-94-0
210 suppliers
$10.00/1g
540-38-5
346 suppliers
$10.00/1g
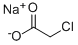
3926-62-3
298 suppliers
$19.73/250g

1878-94-0
210 suppliers
$10.00/1g
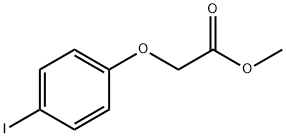
81720-18-5
16 suppliers
$45.00/50mg

1878-94-0
210 suppliers
$10.00/1g