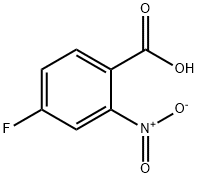
4-Fluoro-2-nitrobenzoic acid synthesis
- Product Name:4-Fluoro-2-nitrobenzoic acid
- CAS Number:394-01-4
- Molecular formula:C7H4FNO4
- Molecular Weight:185.11

446-10-6

394-01-4
The general procedure for synthesizing 2-nitro-4-fluorobenzoic acid from 2-nitro-4-fluorotoluene is as follows: referring to the method of Examples 2-31, a mixture of 4-fluoro-2-nitrotoluene (25.0 g, 161 mmol), potassium permanganate (102 g, 645 mmol), and water (500 mL) was heated to 100 °C and stirred for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and filtered through diatomaceous earth to remove insoluble manganese dioxide produced by the reduction of potassium permanganate. The filtrate was washed with diethyl ether and adjusted to acidity by adding concentrated hydrochloric acid. Subsequently, the organic material in the acidified aqueous phase was extracted with diethyl ether. The organic phases were combined, washed with saturated sodium chloride solution and dried over anhydrous magnesium sulfate. The solvent was removed by evaporation under reduced pressure to give 2-nitro-4-fluorobenzoic acid (10.1 g, 34% yield). The product was confirmed by NMR hydrogen spectroscopy (CDCl3): δ 7.40 (1H, ddd, J = 2.6 Hz, 7.4 Hz, 8.8 Hz), 7.53 (1H, dd, J = 2.6 Hz, 7.6 Hz), 8.00 (1H, dd, J = 5.6 Hz, 8.8 Hz), 8.52 (1H, br s).

80517-21-1
119 suppliers
$8.00/1g

394-01-4
253 suppliers
$8.00/5g
Yield:394-01-4 81%
Reaction Conditions:
with water;hydrogen bromide at 130; for 6.5 h;
Steps:
19.a Example 19; 2-[7-(3-FLUORO-BENZYLOXY)-2-METHYL-4-OXO-4H-QUINAZOLIN-3-YL]-ACETAMIDE; a) 4-Fluoro-2-nitro-benzoic acid
A mixture of 4-fluoro-2-nitro-benzonitrile (12.2 g, 73.7 mmol) suspended in HBr (48%, 74.5 mL, 664 mmol) was heated at 130 °C for 6.5 h. After cooling to rt water (1 L) was added and the resulting precipitate was washed with water and hexane to afford the title compound (11.0 g, 81%) as a light brown solid. MS: M/E = 183.9 (M-H).
References:
F. HOFFMANN-LA ROCHE AG WO2004/54985, 2004, A1 Location in patent:Page 17

446-10-6
331 suppliers
$10.00/25 g

394-01-4
253 suppliers
$8.00/5g
448-39-5
67 suppliers
$27.00/1g

394-01-4
253 suppliers
$8.00/5g
731-86-2
2 suppliers
inquiry

394-01-4
253 suppliers
$8.00/5g

364-78-3
282 suppliers
$7.00/5g

394-01-4
253 suppliers
$8.00/5g