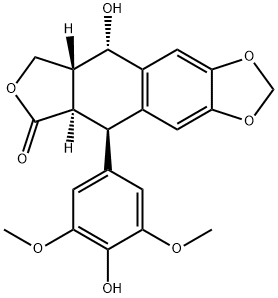
4'-Demethylepipodophyllotoxin synthesis
- Product Name:4'-Demethylepipodophyllotoxin
- CAS Number:6559-91-7
- Molecular formula:C21H20O8
- Molecular Weight:400.38
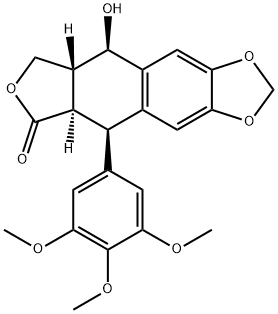
518-28-5

6559-91-7
(5R,5aR,8aR,9R)-9-Hydroxy-5-(3,4,5-trimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8H)-one (onychocercazylidene, 2.1 g, 5.0 mmol) was used as a raw material and dissolved in anhydrous dichloromethane. Sodium iodide (2.3 g, 15.0 mmol) was added to this solution and stirred for 5 min. The reaction mixture was cooled to 0°C (ice-water bath) and methanesulfonic acid (1.48 mL, 15.0 mmol) was added slowly dropwise, after which stirring was continued and gradually brought to room temperature. The reaction was continued at room temperature for 5 hours, during which time nitrogen was introduced to remove the HI gas generated from the reaction. After completion of the reaction, the solvent was removed by concentration under reduced pressure. The crude product was dissolved in a solvent mixture of acetone-water (25 mL-25 mL), barium carbonate (2.0 g, 10.0 mmol) was added, and the reaction was carried out at 40 °C for 30 min. The reaction solution was diluted with dichloromethane (100 mL), poured into 500 mL of 10% aqueous sodium thiosulfate solution and extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent: chloroform:methanol=92:8) to afford the target product (5R,5aR,8aR,9S)-9-hydroxy-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxolan-6(8H)-one ( VII, 1.8g, 90% yield), as white solid.

518-28-5
452 suppliers
$9.00/100mg

6559-91-7
334 suppliers
$5.00/50mg
Yield:6559-91-7 90%
Reaction Conditions:
Stage #1: (-)-podophyllotoxinwith methanesulfonic acid;sodium iodide in dichloromethane at 0 - 20; for 5 h;
Stage #2: with barium carbonate in water monomer;acetone at 40; for 0.5 h;
Steps:
2.4 Example 2: Step Four: Preparationof 4'-O- demethyl podophyllotoxin (V):
The podophyllotoxin 2.1g (5.0mmol) was dissolved in dry methylenechloride, sodium iodide (2.3g, 15.0mmol) Was added to the above solution andstirred for 5 minutes. The reaction solution was cooled to ice-water bath 0 °C, 1.48 Methanesulfonic acid (15.0 mmol) is slowly added dropwise to thereaction mixture and stirring was continued, and then raised to The reactionwas continued at room temperature for 5 hours. Nitrogen, removed HI gasesproduced during the reaction. Then, Reduced pressure in the above reactionsolvent was removed under no processing to the next step. The above crudeproduct Was dissolved in acetone - water (25mL-25mL) mixed solvent was addedbarium carbonate (2.0g, 10.0mmol), For 30 minutes at 40 ° C the reactionconditions, after 100mL of dichloromethane was added to the mixed solution, Thenpour in 500ml of 10% aqueous sodium thiosulfate, and extracted withdichloromethane, the organic phase, dry Dried over sodium sulfate, andconcentrated to give the crude product was purified by silica gelchromatography (eluent: chloroform: methanol = 92: 8), The product was VII1.8g,yield: 90%, white solid.
References:
CN104098594,2016,B Location in patent:Paragraph 0040-0041
![Carbonic acid, 4-[(5R,5aR,8aR)-5,5a,6,8,8a,9-hexahydro-6,9-dioxofuro[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-5-yl]-2,6-dimethoxyphenyl phenylmethyl ester](/CAS/20210111/GIF/156350-44-6.gif)
156350-44-6
0 suppliers
inquiry

6559-91-7
334 suppliers
$5.00/50mg

518-28-5
452 suppliers
$9.00/100mg

6559-91-7
334 suppliers
$5.00/50mg
4375-07-9
67 suppliers
$92.73/250MG
![Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 9-bromo-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)-](/CAS/20210305/GIF/16477-16-0.gif)
16477-16-0
1 suppliers
inquiry

6559-91-7
334 suppliers
$5.00/50mg
![Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-iodo-, (5R,5aR,8aR,9S)-](/CAS/20210305/GIF/746639-24-7.gif)
746639-24-7
1 suppliers
inquiry

6559-91-7
334 suppliers
$5.00/50mg