
4-Cyano-4'-ethylbiphenyl synthesis
- Product Name:4-Cyano-4'-ethylbiphenyl
- CAS Number:58743-75-2
- Molecular formula:C15H13N
- Molecular Weight:207.27
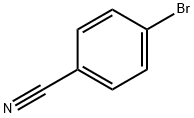
623-00-7
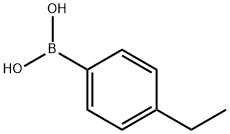
63139-21-9

58743-75-2
GENERAL METHODS: 4-bromobenzonitrile (0.5 mmol), 4-ethylphenylboronic acid (0.75 mmol), Cell-NHC-Pd catalyst (0.75 mol%), K2CO3 (1 mmol), and a solvent mixture of DMF:H2O (1:1, 2.5 mL) were sequentially added to a 25 mL round-bottom flask. Depending on the substrate, the reaction mixture was stirred vigorously at 80 °C for a specific time. Upon completion of the reaction, the solid catalyst was removed by filtration. The filtrate was extracted with ethyl acetate (3 x 5 mL), washed three times sequentially with water and saturated saline, and the organic phase was dried with anhydrous MgSO4. After filtration to remove MgSO4, the solvent was concentrated under reduced pressure to remove the solvent. The crude product was purified by preparative thin layer chromatography (TLC) with the eluent of petroleum ether/ethyl acetate (20:1) to give 4'-ethyl-4-cyanobiphenyl and the yield was calculated.

623-00-7
482 suppliers
$6.00/10g

63139-21-9
290 suppliers
$5.00/250mg

58743-75-2
220 suppliers
$43.70/1g
Yield:58743-75-2 98%
Reaction Conditions:
with potassium carbonate in water;N,N-dimethyl-formamide at 80; for 2.3 h;Suzuki Coupling;
Steps:
2.5. General procedure for the Suzuki reaction of aryl halides witharylboronic acids
General procedure: The aryl halide (0.5 mmol), arylboronic acid (0.75 mmol), Cell-NHC-Pd (0.75 mol%), K2CO3(1 mmol) and DMF:H2O (1:1) (2.5 mL)were successively added into a 25 mL round bottom flask. The mixture was stirred vigorously at 80 °C for the varying specific length of time based on each different substrate. After reaction completion, the solid catalyst was filtered off. The filtrate was extracted with ethyl acetate (3 × 5 mL), washed with water and brine three times, and then dried over anhydrous MgSO4. The MgSO4 was then filtered off. After removal of the solvent, the crude product was purified by preparative TLC (eluent: petroleum ether/ethyl acetate, 20/1) to give the desired product and calculate the yield.
References:
Wang, Xiaoxia;Hu, Peibo;Xue, Fengjun;Wei, Yuping [Carbohydrate Polymers,2014,vol. 114,p. 476 - 483]

63139-21-9
290 suppliers
$5.00/250mg
767-00-0
576 suppliers
$6.00/25g

58743-75-2
220 suppliers
$43.70/1g
623-03-0
474 suppliers
$6.00/25g

63139-21-9
290 suppliers
$5.00/250mg

58743-75-2
220 suppliers
$43.70/1g
25309-64-2
104 suppliers
$9.00/1g

58743-75-2
220 suppliers
$43.70/1g

1585-07-5
319 suppliers
$6.00/5g

58743-75-2
220 suppliers
$43.70/1g