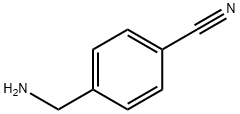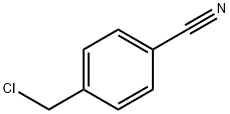
4-(Chloromethyl)tolunitrile synthesis
- Product Name:4-(Chloromethyl)tolunitrile
- CAS Number:874-86-2
- Molecular formula:C8H6ClN
- Molecular Weight:151.59
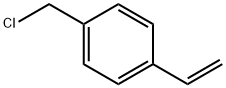
1592-20-7

874-86-2
General procedure for the synthesis of p-cyanobenzyl chloride from 4-chloromethylstyrene: 0.4 mmol of 4-chloromethylstyrene, 2 mmol of sodium nitrite, 5 mg of metal iron(III) porphyrin catalyst, and 4.5 mL of acetonitrile solvent were added to the reaction tube. The mixture was heated to 70 °C under air atmosphere and stirred. 0.5 mL of formic acid was added slowly and dropwise over the first 0.5 hr of the start of the reaction. After the reaction lasted for 4 hours, the heating and stirring was stopped and the reaction mixture was cooled to room temperature. The solvent was removed using a rotary evaporator to obtain the crude product. The crude product was purified by column chromatography and the eluent was a solvent mixture of petroleum ether and ethyl acetate. The final target product, p-chloromethylbenzonitrile, was obtained as a white solid in 96.2% yield. The NMR data are shown below.

1592-20-7
302 suppliers
$11.00/5g

874-86-2
290 suppliers
$11.00/5g
Yield:874-86-2 96.2%
Reaction Conditions:
with formic acid;5,10,15,20‐tetrakis‐(4‐sulfonatophenyl)‐porphyrin‐iron(III) chloride;sodium nitrite in acetonitrile at 70; for 4.5 h;
Steps:
14 Synthesis of chloromethyl benzonitrile
To the reaction tube were added 0.4 mmol of p-chloromethylstyrene, 2 mmol of sodium nitrite,5 mg of metallic iron (III) porphyrin, 4.5 ml of acetonitrile solvent, heated and stirred at 70 ° C in an air atmosphere,0.5 ml of formic acid was added dropwise within the first 0.5 hours, and after 4 hours of reaction, heating and stirring were stopped, and the mixture was cooled to room temperature,The crude product was obtained by rotary evaporator, and then purified by column chromatography to give the desired product,The column eluant used was a mixed solvent of petroleum ether and ethyl acetate.The p-chloromethylbenzonitrile structure is shown below:The compound was a white solid with a yield of 96.2% and its nuclear magnetic data was as follows
References:
CN107573259,2018,A Location in patent:Paragraph 0133-0137
17201-43-3
453 suppliers
$6.00/5g

874-86-2
290 suppliers
$11.00/5g
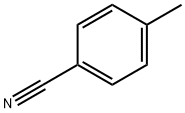
104-85-8
329 suppliers
$11.00/25g

874-86-2
290 suppliers
$11.00/5g
87128-27-6
22 suppliers
inquiry

874-86-2
290 suppliers
$11.00/5g