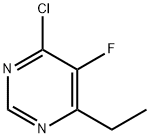
4-Chloro-6-ethyl-5-fluoropyrimidine synthesis
- Product Name:4-Chloro-6-ethyl-5-fluoropyrimidine
- CAS Number:137234-74-3
- Molecular formula:C6H6ClFN2
- Molecular Weight:160.58
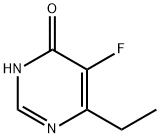
137234-87-8

137234-74-3
Example 1: Synthesis of 4-chloro-6-ethyl-5-fluoropyrimidine <1-1> Preparation of 4-chloro-6-ethyl-5-fluoropyrimidine 80 g of 6-ethyl-5-fluoropyrimidin-4(3H)-one was dissolved in a solvent mixture of 240 mL of dichloromethane and 57.4 mL of N,N-dimethylformamide. Subsequently, 78.24 mL of triethylamine was added to the solution as a base. Phosphoryl chloride (POCl3) was slowly added dropwise to the reaction system over a period of 30 minutes. The reaction mixture was stirred under reflux conditions for 5 hours to ensure complete reaction, after which it was cooled to room temperature. 352 mL of 3N hydrochloric acid solution was carefully added to the reaction mixture at a controlled temperature not exceeding 20°C. The aqueous phase was extracted with 100 mL of dichloromethane and the organic layers were combined and washed with 100 mL of water. The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to afford the oily target product 4-chloro-6-ethyl-5-fluoropyrimidine (85.9 g, 95% yield). 1H-NMR (300 MHz, CDCl3) δ (ppm): 8.70 (1H, s), 2.90 (2H, q), 1.34 (3H, t).

137234-87-8
240 suppliers
$9.00/1g

137234-74-3
336 suppliers
$10.00/1g
Yield:137234-74-3 99%
Reaction Conditions:
with triethylamine;trichlorophosphate in dichloromethane at 48; for 6 h;Product distribution / selectivity;Industry scale;Heating / reflux;
Steps:
2
A reactor was charged with dichloromethane (20 I) and 6-ethyl-5- fluoropyrimidin-4(1 H)-one (5.00 kg, 35.2 mol) at 25-30 0C. The reaction mixture was stirred for 15 min, and triethylamine (3.60 kg, 35.6 mol) was added. Phosphorus oxychloride (5.95 kg, 38.8 mol) was added over 3 h, while the reaction temperature was kept below 35 0C. The reaction mass was heated at reflux (40-48 0C) for 3 h. The mixture was cooled to 5 0C, and
References:
AXELLIA PHARMACEUTICALS APS WO2009/24214, 2009, A1 Location in patent:Page/Page column 3-4

137234-87-8
240 suppliers
$9.00/1g

137234-74-3
336 suppliers
$10.00/1g
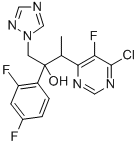
188416-35-5
189 suppliers
$145.00/1g
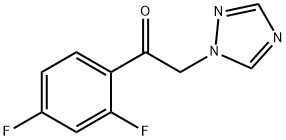
86404-63-9
425 suppliers
$19.00/25g

137234-74-3
336 suppliers
$10.00/1g

137234-85-6
31 suppliers
$420.61/1gm:

137234-74-3
336 suppliers
$10.00/1g
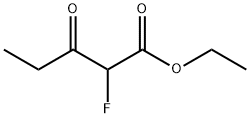
759-67-1
125 suppliers
$41.00/1g

137234-74-3
336 suppliers
$10.00/1g