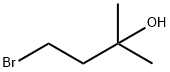
4-BroMo-2-Methylbutan-2-ol synthesis
- Product Name:4-BroMo-2-Methylbutan-2-ol
- CAS Number:35979-69-2
- Molecular formula:C5H11BrO
- Molecular Weight:167.04
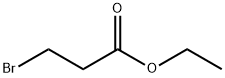
539-74-2
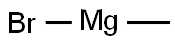
75-16-1

35979-69-2
General procedure for the synthesis of 4-bromo-2-methyl-2-butanol from ethyl 3-bromopropionate and methylmagnesium bromide: To a solution of ethyl 3-bromopropionate (0.2 g, 1.2 mmol) in tetrahydrofuran (THF, 5 mL) was slowly added methylmagnesium bromide (2.4 mL, 2.4 mmol) at 0°C. The reaction mixture was stirred continuously until a thin layer chromatography (TLC) analysis showed complete consumption of the raw material. The reaction mixture was stirred continuously at 0°C until thin layer chromatography (TLC) analysis showed complete consumption of the raw material [Unfolding agent: petroleum ether/ethyl acetate (3:1), Rf value of the product was 0.3]. Upon completion of the reaction, the reaction was quenched with aqueous ammonium chloride (2 mL) at 0°C. The reaction mixture was extracted with ethyl acetate and water by partitioning. The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated to give the crude product. The crude product was purified by column chromatography using petroleum ether/ethyl acetate (3:1) as eluent to give 4-bromo-2-methyl-2-butanol (0.15 g, 75.1% yield) as a yellow oil.

539-74-2
287 suppliers
$5.00/5g
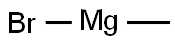
75-16-1
274 suppliers
$12.00/10ml

35979-69-2
77 suppliers
$50.00/50 mg
Yield: 75.1%
Reaction Conditions:
in tetrahydrofuran at 0;
Steps:
45 Intermediate 45 4-bromo-2-methylbutan-2-ol
Intermediate 45 4-bromo-2-methylbutan-2-ol To a solution of methyl 3-bromopropanoate (0.2 g, 1.2 mmol,) in THF (5 mL) was added methyl magnesium bromide (2.4 mL, 2.4 mmol) at 0 °C. The mixture was stirred at this temperature until the substrate was consumed based on tic [petroleum ether/ethyl acetate (3: 1); product Rf 0.3]. The reaction was quenched with ammonium chloride (2 mL) at 0 °C. The mixture was partitioned between ethyl acetate and water. The combined organic layer was dried over sodium sulfate, filtered and concentrated, to give a crude product which was purified by a column chromatography eluting with petroleum ether/ ethyl acetate (3: 1 ) to give 4-bromo-2- methylbutan-2-ol (0.15 g, 75.1 % yield) as a yellow oil.
References:
HYDRA BIOSCIENCES, INC.;CHENARD, Bertrand;GALLASCHUN, Randall WO2014/143799, 2014, A2 Location in patent:Page/Page column 403
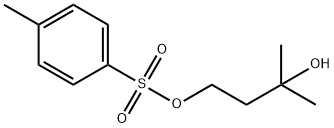
17689-66-6
26 suppliers
$547.00/5g

35979-69-2
77 suppliers
$50.00/50 mg
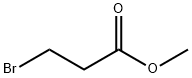
3395-91-3
268 suppliers
$5.00/5g
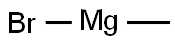
75-16-1
274 suppliers
$12.00/10ml

35979-69-2
77 suppliers
$50.00/50 mg

539-74-2
287 suppliers
$5.00/5g

35979-69-2
77 suppliers
$50.00/50 mg
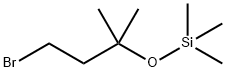
67883-17-4
1 suppliers
inquiry

35979-69-2
77 suppliers
$50.00/50 mg