
4-BENZYLOXY-3-METHOXYPHENYLACETONITRILE synthesis
- Product Name:4-BENZYLOXY-3-METHOXYPHENYLACETONITRILE
- CAS Number:1700-29-4
- Molecular formula:C16H15NO2
- Molecular Weight:253.3

4468-59-1

100-39-0

1700-29-4
4-Phenylmethoxy-3-methoxyphenylacetonitrile was prepared by reaction of 4-hydroxy-3-methoxyphenylacetonitrile (1.08 g, 6.60 mmol, 1.0 eq.) and benzyl bromide (1.24 g, 7.26 mmol, 1.1 eq.) in acetone (14 mL) according to the modified method of Imbri et al. First, K2CO3 (1.10 g, 7.92 mmol, 1.2 eq.) was added to the acetone solution of 4-hydroxy-3-methoxyphenylacetonitrile and the suspension was stirred for 10 min. Subsequently, benzyl bromide was added slowly dropwise via syringe. The reaction mixture was heated to reflux for 4 hours. Upon completion of the reaction, the suspension was separated by filtration and the precipitated solid was washed with acetone (10 mL). The filtrate was concentrated under reduced pressure and diluted with dichloromethane (DCM, 50 mL). The organic layer was washed sequentially with 2N NaOH (30 mL) and brine (30 mL) and then dried with Na2SO4. Finally, the solvent was removed by vacuum to afford the target product, 4-phenylmethoxy-3-methoxyphenylacetonitrile, as a yellow solid (1.67 g, 6.59 mmol, quantitative yield). The thin layer chromatography (TLC) Rf value of the product was 0.43 (cyclohexane/ethyl acetate, 2:1). The melting point was 65-66 °C. Nuclear magnetic resonance hydrogen (1H NMR, 400 MHz, CDCl3) and carbon (13C NMR, 100.6 MHz, CDCl3) data were consistent with the literature reports, confirming the structure of the product.

4468-59-1
75 suppliers
$23.00/250 mg

100-39-0
438 suppliers
$10.00/10 g

1700-29-4
90 suppliers
$10.00/250mg
Yield: 100%
Reaction Conditions:
Stage #1:4-hydroxy-3-methoxyphenylacetonitrile with potassium carbonate in acetone for 0.166667 h;
Stage #2:benzyl bromide in acetone for 4 h;Reflux;
Steps:
7 7.3.7
4-(Benzyloxy)-3-methoxyphenylacetonitrile (11)
The title compound was prepared according to a modified procedure of Imbri et al.
12
To a solution of 4-hydroxy-3-methoxyphenylacetonitrile (10, 1.08 g, 6.60 mmol, 1.0 eq.) in acetone (14 mL), K2CO3 (1.10 g, 7.92 mmol, 1.2 eq.) was added.
The suspension was stirred for 10 min.
Subsequently, benzyl bromide (1.24 g, 7.26 mmol, 1.1 eq.) was added dropwise with a syringe.
The reaction mixture was heated to reflux for 4 h.
The suspension was filtered with suction the precipitated solid was washed with acetone (10 mL).
The filtrate was concentrated under reduced pressure, the remaining solution was diluted with DCM (50 mL).
The organic layers were washed with 2 N NaOH (30 mL), brine (30 mL) and dried over Na2SO4.
The solvent was removed in vacuo to furnish the title compound as a yellow solid (1.67 g, 6.59 mmol, quant.).
Yield: 1.67 mg (6.59 mmol, 100 % Lit.).
Rf = 0.43 (cyclohexane/ethyl acetate, 2:1).
Mp: 65-66 °C.
1H NMR, COSY, NOESY (400 MHz, CDCl3): δ = 7.44-7.29 (m, 5H, Ph), 6.86 (d, 3J = 8.2 Hz, 1H, 5-H), 6.84 (d, 4J = 2.1 Hz, 1H, 2-H), 6.78 (dd, 3J = 8.2 Hz, 4J = 2.1 Hz, 1H, 6-H), 5.15 (s, 2H, PhCH2O), 3.90 (s, 3H, OCH3), 3.68 (s, 2H, ArCH2CN) ppm.
13C NMR (100.6 MHz, CDCl3): δ = 150.2 (Cq-3), 148.0 (Cq-4), 136.9 (Cq-1, Ph), 128.7 (CH-3, CH-5, Ph), 128.0 (CH-4, Ph), 127.3 (CH-2, CH-6, Ph), 122.8 (Cq-1), 120.3 (CH-6), 118.2 (CN), 114.4 (CH-2), 111.6 (CH-5), 71.2 (PhCH2O), 56.2 (OCH3), 23.3 (ArCH2CN) ppm.
The spectroscopic data matched those reported in the literature.
42
References:
Colligs, Vanessa;Hansen, Steven Peter;Imbri, Dennis;Seo, Ean-Jeong;Kadioglu, Onat;Efferth, Thomas;Opatz, Till [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 22,p. 6137 - 6148]

2426-87-1
251 suppliers
$8.00/5g

1700-29-4
90 suppliers
$10.00/250mg
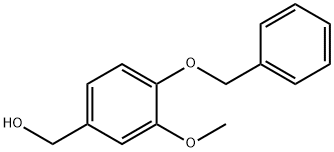
33693-48-0
69 suppliers
$18.00/1 g

1700-29-4
90 suppliers
$10.00/250mg

100-39-0
438 suppliers
$10.00/10 g

1700-29-4
90 suppliers
$10.00/250mg
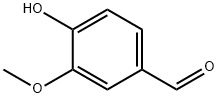
121-33-5
1170 suppliers
$9.00/1g

1700-29-4
90 suppliers
$10.00/250mg