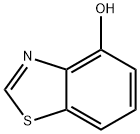
4-Benzothiazolol synthesis
- Product Name:4-Benzothiazolol
- CAS Number:7405-23-4
- Molecular formula:C7H5NOS
- Molecular Weight:151.19

3048-46-2

7405-23-4
General procedure for the synthesis of 4-hydroxybenzothiazole from 4-methoxybenzothiazole: Boron bromide (3.09 mL, 1M DCM solution, 3.09 mmol) was slowly added dropwise to a stirring solution of 4-methoxybenzothiazole (510 mg, 3.09 mmol) in anhydrous dichloromethane (30 mL) at 0 °C. The reaction mixture was gradually warmed to 40 °C and stirred continuously overnight. Upon completion of the reaction, the solution was concentrated under vacuum and subsequently diluted with water and 2N hydrochloric acid. The aqueous phase was neutralized to pH 7 with sodium bicarbonate and then extracted with ethyl acetate (3 x 100 mL). The organic extracts were combined, dried with magnesium sulfate, and the solvent was removed under vacuum. The resulting oil was purified by fast chromatography on a silica gel column with the eluent ratio tapered from hexane:ethyl acetate [4:1] to hexane:ethyl acetate [7:3], resulting in 4-hydroxybenzothiazole as a brown solid (730 mg, 80% yield). The NMR hydrogen spectrum (300 MHz, CDCl3) data were as follows: δ 7.59 (1H, s, hydrogen on the thiazole ring), 7.46 (1H, dd, aromatic hydrogen), 7.36 (1H, t, aromatic hydrogen), 7.02 (1H, dd, aromatic hydrogen).

3048-46-2
49 suppliers
$150.00/250mg

7405-23-4
106 suppliers
$23.00/100mg
Yield: 80%
Reaction Conditions:
with boron tribromide in 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran; pH=7 at 40;
Steps:
4-Hydroxy-benzothiazole
4-Hydroxy-benzothiazole Boron bromide (3. 09ML, 1M solution in DCM, 3.09 mmol) was added dropwise at 0 C to a stirred solution OF 4-METHOXY-BENZOTHIAZOLE (510 mg, 3.09 mmol) in dry DCM (30ML). The resulting solution was warmed to 40 C and allowed to stir overnight. The resulting solution was concentrated IIZ vacuo and diluted with water and HCl (2N). The aqueous phase was neutralised to pH-7 with NaHC03 and the solution extracted with EtOAc (3 x 100 ml) and the combined organic extracts dried (MGS04) and the solvent removed III vacuo. The resulting oil was purified by flash chromatography eluting silica gel with hexane: EtOAc [4: 1] to hexane: EtOAc [7: 3] to give the title compound as a tan solid (730mg, 80%) ; ON (300 MHz, CDC13) 7.59 (1H, s, C$, 7.46 (1H, dd, Ar), 7.36 (1H, t, Ar), 7.02 (1H, dd, Ar).
References:
ELI LILLY AND COMPANY WO2004/43903, 2004, A1 Location in patent:Page 68 - 69

5464-79-9
203 suppliers
$19.00/250mg

7405-23-4
106 suppliers
$23.00/100mg
3507-27-5
92 suppliers
$65.00/25mg

7405-23-4
106 suppliers
$23.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

7405-23-4
106 suppliers
$23.00/100mg
30740-99-9
1 suppliers
inquiry

7405-23-4
106 suppliers
$23.00/100mg