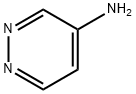
4-AMINOPYRIDAZINE synthesis
- Product Name:4-AMINOPYRIDAZINE
- CAS Number:20744-39-2
- Molecular formula:C4H5N3
- Molecular Weight:95.1
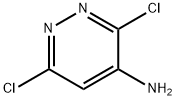
823-58-5

20744-39-2
General procedure for the synthesis of 4-aminopyridazine from 3,6-dichloropyridazin-4-amine: 1. 3,6-dichloropyridazin-4-amine (5.00 g, 18.2 mmol) was dissolved in tetrahydrofuran (100 ml), sodium hydroxide (8.00 g, 200 mmol) and water (32 ml) were added. 2. 10% palladium-carbon catalyst (500 mg) was added to the above mixture and the reaction was stirred for 2 days at room temperature under hydrogen atmosphere. 3. After completion of the reaction, the insoluble material was removed by filtration and the filtrate was concentrated. 4. 4. The concentrated residue was dissolved in methanol (100 ml), filtered again to remove the insoluble material, and the filtrate was concentrated to obtain the target product 4-aminopyridazine as a solid. Product characterization: 1H-NMR (DMSO-d6) δ; 2.51 (2H, br s), 6.00 (1H, br s), 7.81-7.85 (1H, m), 7.98-8.00 (1H, m).

823-58-5
151 suppliers
$9.00/1g

20744-39-2
176 suppliers
$15.00/250mg
Yield:20744-39-2 100%
Reaction Conditions:
with sodium hydroxide;hydrogen;palladium 10% on activated carbon in tetrahydrofuran;water at 20; for 48 h;
Steps:
151.1
Example 151; 4-(3-Phenyl-1,2,4-thiadiazol-5-yl)-N-pyridazin-4-ylpiperazine-1-carboxamide; (1) Pyridazine-4-amine; A mixture of 3,6-dichloropyridazine-4-amine (5.00 g, 18.2 mmol), tetrahydrofuran (100 ml), sodium hydroxide (8.00 g, 200 mmol), water (32 ml) and 10% palladium-carbon (500 mg) was stirred under a hydrogen atmosphere at room temperature for 2 days, insolubles were filtered off and the filtrate was concentrated. The residue was dissolved in methanol (100 ml), insolubles were filtered off and the filtrate was concentrated to obtain the desired product quantitatively as a solid. 1H-NMR (DMSO-d6) δ; 2.51 (2H, br s), 6.00 (1H, br s), 7.81 - 7.85 (1H, m), 7.98 - 8.00 (1H, m).
References:
EP1813606,2007,A1 Location in patent:Page/Page column 83-84
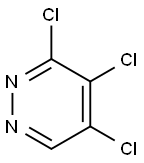
14161-11-6
151 suppliers
$9.00/1g

20744-39-2
176 suppliers
$15.00/250mg
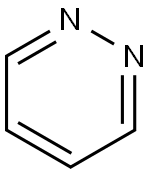
289-80-5
244 suppliers
$6.00/1g

20744-39-2
176 suppliers
$15.00/250mg
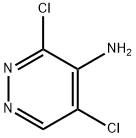
53180-76-0
87 suppliers
$61.00/100mg

20744-39-2
176 suppliers
$15.00/250mg
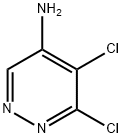
89180-50-7
62 suppliers
$39.00/100mg

20744-39-2
176 suppliers
$15.00/250mg