
4-Amino-3-nitropyridine synthesis
- Product Name:4-Amino-3-nitropyridine
- CAS Number:1681-37-4
- Molecular formula:C5H5N3O2
- Molecular Weight:139.11
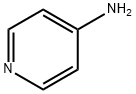
504-24-5

1681-37-4
The general procedure for the synthesis of 4-amino-3-nitropyridine from 4-aminopyridine was as follows: pyridin-4-amine (5.0 g, 50.0 mmol) was dissolved in concentrated sulfuric acid (20 mL) under ice-bath conditions. Keeping the reaction temperature in the range of 0-10 °C, fuming nitric acid (2.5 mL) was added slowly and dropwise. After the dropwise addition, the reaction was continued to be stirred at 0-10 °C for 5 hours. Subsequently, the reaction mixture was warmed to room temperature and heated at 90°C for 3 hours. After the reaction was completed, the mixture was continued to be stirred at room temperature overnight. The reaction mixture was slowly poured into ice water and the pH was adjusted with ammonia to 7. The resulting precipitate was collected by filtration and dried under reduced pressure to afford 4-amino-3-nitropyridine as a yellow solid (5.1 g, 70% yield). Mass spectrometry analysis showed [M + H]+ m/z: 140.04.
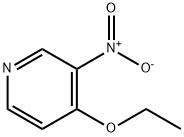
1796-84-5
167 suppliers
$9.00/250mg

1681-37-4
344 suppliers
$5.00/1g
Yield:1681-37-4 75%
Reaction Conditions:
with ammonium acetate;triethylamine in phosphorus pentaoxide;ethyl acetate
Steps:
2 Preparation of 3-Nitro-4-aminopyridine
EXAMPLE 2 Preparation of 3-Nitro-4-aminopyridine A 25 ml round bottom flask was fitted with a reflux condenser and magnetic stirrer and placed in an oil bath. The flask was charged with 1.0 g (5.95 mmol) of 4-ethoxy-3-nitropyridine and 5.0 g (65 mmol) of ammonium acetate. The reaction mixture was heated at 120° C. to provide a homogeneous liquid. The progress of the reaction was followed by thin layer chromotography employing a 10:1 ethyl acetate:triethylamine solvent system. After 21/2 hours the reaction mixture was cooled and poured into water. The yellow precipitate was collected by filtration, washed with water and dried in vacuo at 60° C. over phosphorus pentoxide. A total of 620 mg of 3-nitro-4-aminopyridine was obtained and chromatographically verified by comparison to an authentic reference standard. Yield 75%.
References:
Eli Lilly and Company US4552967, 1985, A

504-24-5
487 suppliers
$10.00/1 g

1681-37-4
344 suppliers
$5.00/1g
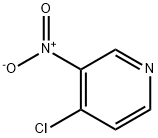
13091-23-1
341 suppliers
$14.14/1gm:

1681-37-4
344 suppliers
$5.00/1g
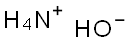
1336-21-6
733 suppliers
$14.56/250ML
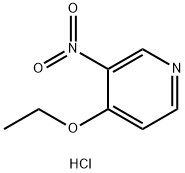
94602-04-7
72 suppliers
$37.79/5g

1681-37-4
344 suppliers
$5.00/1g

94602-04-7
72 suppliers
$37.79/5g

1681-37-4
344 suppliers
$5.00/1g