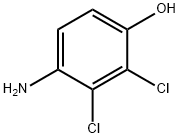
4-Amino-2,3-dichlorphenol synthesis
- Product Name:4-Amino-2,3-dichlorphenol
- CAS Number:39183-17-0
- Molecular formula:C6H5Cl2NO
- Molecular Weight:178.02
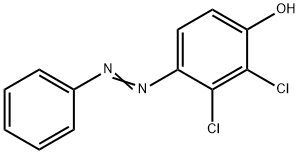
201656-71-5

39183-17-0
To a 100 mL two-necked flask equipped with a stirrer and reflux condenser was added 8.0 g (0.03 mol) of 2,3-dichloro-4-phenylazophenol (CAS: 201656-71-5) and 1 g of sodium hydroxide dissolved in 40 mL of ethanol. The reaction system was cooled in an ice-water bath, followed by the addition of 4.5 g (0.12 mol) of sodium borohydride in one drop. After the dropwise addition was completed, the ice bath was removed and the reaction mixture was gradually warmed up to room temperature and the reaction was continued with stirring for 4 hours. Upon completion of the reaction, the pH of the reaction solution was adjusted to 7 with dilute hydrochloric acid solution and then extracted three times with ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate and the solvent was removed by distillation under reduced pressure. The crude product was recrystallized with 20 mL of toluene, and after filtration and drying, 5.08 g of light pink crystalline 4-amino-2,3-dichlorophenol was obtained in 95% yield and 100% purity.

111-48-8
0 suppliers
$38.70/100g
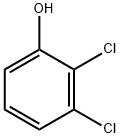
576-24-9
253 suppliers
$14.00/1g

39183-17-0
71 suppliers
$22.00/100mg
Yield:39183-17-0 83%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;hydrogen;aniline;sodium nitrite;aluminum nickel in methanol;water
Steps:
6 EXAMPLE 6
EXAMPLE 6 At 0 to 5° C., a solution of 3.8 g (0.055 mol) of NaNO2 in 6 ml of H2 O is added dropwise to 25 ml of aqueous HCl (15%) and 4.9 g (0.0525 mol) of aniline in a 100 ml two-neck flask fitted with internal thermometer and magnetic stirrer. After 10 minutes, this diazonium salt solution is added at 5 to 10° C. to a solution of 8.15 g (0.05 mol) of 2,3-dichlorophenol and 10 g (0.25 mol) of NaOH in 100 ml of H2 O. The mixture is allowed to warm to room temperature, stirred for a further 1 hour and neutralized with aqueous HCl (pH=7). In a 500 ml three-neck flask fitted with internal thermometer, stirrer and gas inlet tube, this solution is admixed with 300 ml of methanol, 2.5 g of Raney nickel and 15 μl of bis(2-hydroxyethyl) sulphide, and the flask is flushed with argon. At 40 to 50° C., 2300 ml of hydrogen (0.1 mol) are then applied with a pressure of about 1 bar over 1.5 hours. The reaction mixture is admixed with a little activated charcoal, stirred for a further 15 minutes and filtered over a bed of silica gel. The methanol is distilled off and pure 4-hydroxy-2,3-dichloroaniline precipitates from the aqueous solution, giving a yield of 83%.
References:
Bayer Aktiengesellschaft US6124504, 2000, A

201656-71-5
0 suppliers
inquiry

39183-17-0
71 suppliers
$22.00/100mg

576-24-9
253 suppliers
$14.00/1g

39183-17-0
71 suppliers
$22.00/100mg
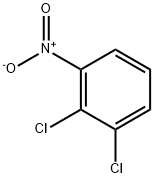
3209-22-1
19 suppliers
$10.00/1g

39183-17-0
71 suppliers
$22.00/100mg