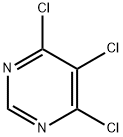
4,5,6-Trichloropyrimidine synthesis
- Product Name:4,5,6-Trichloropyrimidine
- CAS Number:1780-27-4
- Molecular formula:C4HCl3N2
- Molecular Weight:183.42
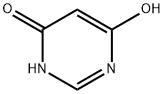
1193-24-4

1780-27-4
The general procedure for the synthesis of 4,5,6-trichloropyrimidine from 4,6-dihydroxypyrimidine was as follows: 89.7 g of 4,6-dihydroxypyrimidine and 179.3 g of chlorobenzene were added to a 1000 mL four-neck flask. The mixture was heated to 40 °C, and then 129.6 g of sulfonyl chloride was slowly added dropwise over a period of 1 hour. After the dropwise addition was completed, the reaction mixture was kept stirred at 40 °C for 6 hours. Subsequently, 269.9 g of phosphoryl chloride was added to the reaction mixture at the same temperature. Next, 178.1 g of triethylamine was slowly added dropwise over a period of 2 hours at temperatures ranging from 40 to 80 °C. After completion of the dropwise addition, the reaction mixture was warmed up to 83 °C and kept for 10 hours. Upon completion of the reaction, the mixture was cooled to room temperature. To another 100 mL four-necked flask was added 269.0 g of water and heated to 40 °C. The above reaction mixture was slowly added dropwise to the water over a period of 30 minutes, keeping the internal temperature between 30 and 50 °C during the dropwise addition. Upon completion of the reaction, the mixture was filtered using Radiolite (registered trademark) to separate the organic and aqueous layers. The aqueous layer was extracted with 44.8 g of chlorobenzene and the organic layers were combined. The combined organic layers were washed with 44.8 g of water and subsequently concentrated under reduced pressure to give 169.2 g of black oil. The oily substance was analyzed by high performance liquid chromatography (HPLC) with internal standard method and it was determined that the oily substance contained 126.9 g of 4,5,6-trichloropyrimidine in 86% yield.

1193-24-4
568 suppliers
$6.00/25g

1780-27-4
149 suppliers
$11.00/100mg
Yield:1780-27-4 86%
Reaction Conditions:
with sulfuryl dichloride;triethylamine;trichlorophosphate in chlorobenzene at 40 - 83; for 19 h;Product distribution / selectivity;
Steps:
2
Example 2; To a 1000 mL four-neck flask, 89.7 g of 4,6-dihydroxypyrimidine and 179.3 g of chlorobenzene were added. The obtained mixture was adjusted at 40°C, and then, 129.6 g of sulfuryl chloride was added dropwise thereto over 1 hour. The obtained mixture was maintained at the same temperature for 6 hours. To the obtained reaction mixture, 269.9 g of phosphorus oxychloride was added at the same temperature. Further, 178.1 g of triethylamine was added dropwise thereto over 2 hours at an inner temperature of 40 to 80°C. After completion of the addition, the obtained mixture was maintained at 83°C for 10 hours. The obtained reaction mixture was cooled to room temperature. To another 100 mL four-neck flask, 269.0 g of water was added followed by adjusting at 40°C. To it, the obtained reaction mixture was added dropwise over 30 minutes. The inner temperature during the addition was 30 to 50°C. The obtained mixture was filtrated using Radiolite (registered trademark) and the obtained filtrate was separated to an organic layer and an aqueous layer. The aqueous layer was extracted with 44.8 g of chlorobenzene, and the obtained chlorobenzene layer was mixed with the previously obtained organic layer. The organic layer after mixing was washed with 44.8 g of water and then, concentrated under reduced pressure to obtain 169.2 g of black oily matter. The oily matter was analyzed by high performance liquid chromatography internal standard method, and 126.9 g of 4,5,6-trichlorpyrimidine was containd in the oily matter. The yield was 86%.
References:
EP2128141,2009,A1 Location in patent:Page/Page column 7
4316-93-2
286 suppliers
$12.00/5g

1780-27-4
149 suppliers
$11.00/100mg
1193-56-2
44 suppliers
$78.00/100mg

1780-27-4
149 suppliers
$11.00/100mg
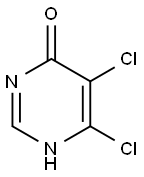
88982-91-6
54 suppliers
$75.00/250mg

1780-27-4
149 suppliers
$11.00/100mg
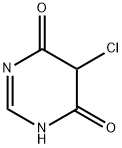
63447-41-6
3 suppliers
inquiry

1780-27-4
149 suppliers
$11.00/100mg