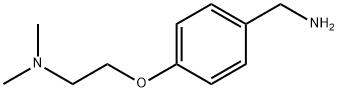
4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE synthesis
- Product Name:4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE
- CAS Number:20059-73-8
- Molecular formula:C11H18N2O
- Molecular Weight:194.27
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZALDEHYDE](/CAS/GIF/15182-92-0.gif)
15182-92-0
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE](/CAS/GIF/20059-73-8.gif)
20059-73-8
General procedure for the synthesis of 4-[2-(dimethylamino)ethoxy]benzylamine from 4-(2-(dimethylamino)ethoxy)benzenecarboxaldehyde: 27 kg of 80% acetic acid was added to the reactor, followed by 5.3 kg of hydroxylamine hydrochloride. The mixture was cooled to 0-5 °C. A solution of acetic acid containing 13.5 kg of 100% 4-(2-dimethylaminoethoxy)benzaldehyde was added to the above cooled mixture. The reaction mixture was kept at 0-5°C for at least 2 hours. Upon completion of the reaction, 10.8 kg of powdered zinc was added in batches and the temperature was controlled not to exceed 50°C. The mixture was stirred at 45-50°C for at least 5 hours and then heated to 65-70°C and kept for at least 2 hours. Upon completion of the reaction, the reaction mixture was distilled to give a thick but stirrable residue. To the residue was added 20.3 kg of deionized water and the mixture was cooled to 20-30 °C. 54 kg of 30% ammonia was added slowly and dropwise to ensure that the pH of the reaction mixture was above 9.5. After stirring for at least 10 minutes at 20-30 °C, the reaction mixture was extracted with 40.5 kg of dichloromethane at 20-25 °C. The reaction mixture was then extracted with aqueous phase of 30% ammonia. The organic phase was separated and the aqueous phase was then subjected to a second extraction with 27 kg of dichloromethane, stirring for at least 5 minutes before combining the organic phases. The solvent was removed by distillation to give an oily residue. To the residue was added 54 kg of sec-butanol and 20.3 kg of N,N-dimethylformamide to give a solution containing 11.5 kg of 4-(2-dimethylaminoethoxy)benzylamine in 84.7% yield.
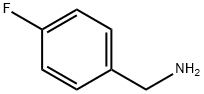
140-75-0
482 suppliers
$10.00/10g
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE](/CAS/GIF/20059-73-8.gif)
20059-73-8
248 suppliers
$26.00/1g
Yield:20059-73-8 91%
Reaction Conditions:
Stage #1:2-(N,N-dimethylamino)ethanol with sodium hydride at 130 - 140; for 1 h;
Stage #2:para-fluorobenzylamine at 130 - 140; for 5 h;Product distribution / selectivity;
Steps:
1
[Example 1]; Preparation of 4- [2- (dimethyl amino)ethoxy Ibenzylamine; 2.54g(63.43mmol) of 60% sodium hydride was slowly dropwised to6.63g(74.37mmol) of 2-(dimethylamino)ethanol at 0°C.After finishing the drop wise, the temperature of reactor was raised to 130~140°Cand mixed for 1 hour. 5.48g(43.79mmol) of 4-fluorobenzylamine was slowlydropwised therein, followed by mixing at 130~140°C for 5 hours. After finishing thereaction, the reactant was cooled down to room temperature. 100mNo. of H O was addedthereto, followed by mixing for 30 minutes, and then extracted withchloroform(150mNo.x2), and dried with magnesium sulfate anhydrous, which was thenfiltered, and 7.74g(91% yield) of a desired product was obtained by decompressing -distilling.,ppm): 1.63(br,NH ), 2.31(s,6H), 2.67~2.72(t,2H), 3.77(s,2H),4.00~4.05(t,2H), 6.84~6.89(d,2H), 7.17~7.21(d,2H)
References:
IL YANG PHARM. CO., LTD WO2006/11696, 2006, A1 Location in patent:Page/Page column 7-8
![Ethanamine, 2-[4-(chloromethyl)phenoxy]-N,N-dimethyl-](/CAS/20210305/GIF/131028-55-2.gif)
131028-55-2
0 suppliers
inquiry
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE](/CAS/GIF/20059-73-8.gif)
20059-73-8
248 suppliers
$26.00/1g
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZONITRILE](/CAS/GIF/24197-95-3.gif)
24197-95-3
41 suppliers
inquiry
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE](/CAS/GIF/20059-73-8.gif)
20059-73-8
248 suppliers
$26.00/1g
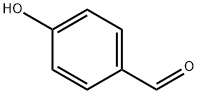
123-08-0
960 suppliers
$5.00/10g
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE](/CAS/GIF/20059-73-8.gif)
20059-73-8
248 suppliers
$26.00/1g
149505-94-2
41 suppliers
$39.00/250mg
![4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE](/CAS/GIF/20059-73-8.gif)
20059-73-8
248 suppliers
$26.00/1g