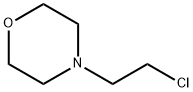
4-(2-Chloroethyl)morpholine synthesis
- Product Name:4-(2-Chloroethyl)morpholine
- CAS Number:3240-94-6
- Molecular formula:C6H12ClNO
- Molecular Weight:149.62

622-40-2

3240-94-6
Thionyl chloride (69.44 mmol, 5.06 mL, 5.0 eq.) was slowly added to a stirred solution of 2-morpholinoethan-1-ol (13.88 mmol, 2.5 g, 1.0 eq.) in dichloromethane (25 mL) with a catalytic amount of DMF at 0 °C. The reaction mixture was heated to 40 °C overnight. The reaction progress was monitored by TLC. After completion of the reaction, the solvent was removed by vacuum evaporation to give the crude product. The crude product was diluted with dichloromethane and washed with saturated sodium bicarbonate solution. The organic layer was concentrated under reduced pressure to give the crude residue. The crude product was purified by column chromatography using dichloromethane solution containing 3% methanol as eluent to afford the target compound 4-(2-chloroethyl)morpholine (2.0 g, 74.0% yield) as a colorless liquid.1H NMR (DMSO-d6, 300 MHz): δ 3.69 (t, J = 6.9 Hz, 2H), 3.57 (m, J = 4.5 Hz, 4H ), 2.64 (t, J = 6.9 Hz, 2H), 2.43 (t, J = 4.5 Hz, 4H); MS: [M + H]+ 149.95 (10%).

622-40-2
354 suppliers
$5.00/25g

3240-94-6
129 suppliers
$27.00/1g
Yield:3240-94-6 74%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide in dichloromethane at 0 - 40;
Steps:
2.2 Synthesis of 4-(2-chloroethyl)morpholine
To a stirred solution of 2-morpholinoethan-l-ol (step 1, 2.5 g, 13.88 mmol, 1.0 eq) in DCM (25 ml) at 0 °C then added thionyl chloride (5.06 mL, 69.44 mmol, 5.0 eq) followed by DMF (Cat.). The reaction mixture was heated to 40 °C for overnight. After completion of the reaction (monitored by TLC), the solvent was evaporated in vacuo providing a crude residue, which was diluted with DCM and washed with saturated sodium bicarbonate solution. Organic layer was concentrated under reduced preesure gave crude residue which was purified by column chromatography by using 3% MeOH in DCM as an eluent to afford the desired compound (2.0 g, yield: 74.0%) as a colour less liquid. 1H NMR (DMSO-d6, 300MHz): δ 3.69 (t, J = 6.9 Hz, 2H), 3.57 (m, J = 4.5 Hz, 4H), 2.64 (t, J = 6.9 Hz, 2H) and 2.43 (t, J = 4.5 Hz, 4H); Mass: [M+H]+149.95 (10%).
References:
HETERO LABS LIMITED;BANDI, Parthasaradhi Reddy;KURA, Rathnakar Reddy;GAZULA LEVI, David Krupadanam;ADULLA, Panduranga Reddy;MUKKERA, Venkati;NEELA, Sudhakar WO2017/149518, 2017, A1 Location in patent:Page/Page column 25; 26
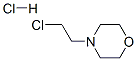
3647-69-6
366 suppliers
$5.00/10g

3240-94-6
129 suppliers
$27.00/1g
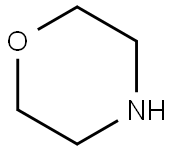
110-91-8
707 suppliers
$9.00/1g

107-06-2
440 suppliers
$14.00/25g

3240-94-6
129 suppliers
$27.00/1g
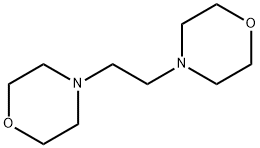
1723-94-0
12 suppliers
inquiry

