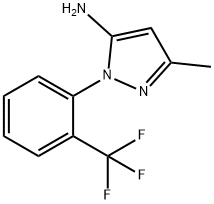
3-METHYL-1-[2-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-5-AMINE synthesis
- Product Name:3-METHYL-1-[2-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-5-AMINE
- CAS Number:380238-10-8
- Molecular formula:C11H10F3N3
- Molecular Weight:241.21
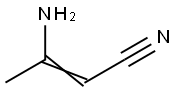
1118-61-2
![1-[2-(Trifluoromethyl)phenyl]hydrazine](/CAS/GIF/365-34-4.gif)
365-34-4
![3-METHYL-1-[2-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-5-AMINE](/StructureFile/ChemBookStructure5/GIF/CB1667679.gif)
380238-10-8
GENERAL METHOD: 3-Aminobut-2-enenenitrile (10 mmol) was added to a suspension of (2-(trifluoromethyl)phenyl)hydrazine (10 mmol) in 12 M HCl-H2O (12 mL, 1:3 v/v). The reaction mixture was heated to reflux for 12 h, subsequently cooled to room temperature and neutralized with 2.5 M NaOH solution. The reaction mixture was extracted with dichloromethane (3 x 30 mL). The organic phases were combined, washed with saturated saline (20 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was ground with hexane to give a solid product, which was separated by filtration. If necessary, the product was purified by column chromatography.

1118-61-2
256 suppliers
$9.00/25g
![1-[2-(Trifluoromethyl)phenyl]hydrazine](/CAS/GIF/365-34-4.gif)
365-34-4
80 suppliers
$30.00/1g
![3-METHYL-1-[2-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-5-AMINE](/StructureFile/ChemBookStructure5/GIF/CB1667679.gif)
380238-10-8
44 suppliers
$65.00/250mg
Yield:380238-10-8 73%
Reaction Conditions:
Stage #1: 3-Aminocrotononitrile;2-(trifluoromethyl)phenylhydrazinewith hydrogenchloride in water; for 12 h;Reflux;
Stage #2: with sodium hydroxide in water;
Steps:
General Procedure for the Synthesis of 1-Substituted-3-methyl-1H-pyrazol-5-amines 46a-l.
General procedure: 3-aminocrotononitrile (48, 10 mmol) was added to a suspension of the appropriate arylhydrazine 49a-l (10 mmol) in 12M HCl-H2O (12 mL, 1:3), and the resulting mixture was heated under reflux for 12 hours and then cooled and neutralized with 2.5M sodium hydroxide solution. The suspension was extracted with CH2Cl2 (3x30 mL). The organic phases were washed with brine (20 mL), dried and the solvent evaporated in vacuo. The residue oil was triturated with hexane to obtain a solid which was separated by filtration. Subsequent chromatography was performed where indicated.
References:
Marinozzi, Maura;Carotti, Andrea;Sansone, Emanuele;MacChiarulo, Antonio;Rosatelli, Emiliano;Sardella, Roccaldo;Natalini, Benedetto;Rizzo, Giovanni;Adorini, Luciano;Passeri, Daniela;De Franco, Francesca;Pruzanski, Mark;Pellicciari, Roberto [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 11,p. 3429 - 3445] Location in patent:supporting information; experimental part
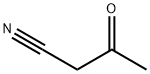
2469-99-0
94 suppliers
$16.00/1g
![1-[2-(Trifluoromethyl)phenyl]hydrazine](/CAS/GIF/365-34-4.gif)
365-34-4
80 suppliers
$30.00/1g
![3-METHYL-1-[2-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-5-AMINE](/StructureFile/ChemBookStructure5/GIF/CB1667679.gif)
380238-10-8
44 suppliers
$65.00/250mg