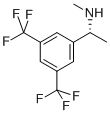
(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine synthesis
- Product Name:(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine
- CAS Number:334477-60-0
- Molecular formula:C11H11F6N
- Molecular Weight:271.2
![Benzenemethanamine, N,α-dimethyl-N-[(1S)-1-phenylethyl]-3,5-bis(trifluoromethyl)-, (αR)-](/CAS/20210305/GIF/672907-02-7.gif)
672907-02-7
0 suppliers
inquiry
![(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/334477-60-0.gif)
334477-60-0
39 suppliers
$95.00/250mg
Yield:334477-60-0 92%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in methanol at 60; under 3750.38 Torr; for 17.5 h;
Steps:
11.b Step (b), hydrogenolysis
Step (b), hydrogenolysis. To 2.4 ml of methanol, 0.90 g (2.40 mmol, 1eq.) of the above purified product of the optically active tertiary amine and 18.0 mg (0.05wt% Pd) of a palladium catalyst (having 5% palladium carried on an activated carbon containing 50wt% of water) were added. The hydrogen pressure was adjusted to 0.5 MPa, and the stirring was conducted at 60°C for 17.5 hr. After the reaction, the reaction liquid was filtrated using a filtration aid (CELITE (trade name)), concentrated, and vacuum-dried, thereby obtaining 0.60g of a crude product of an optically active (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethylamine N-monomethyl, represented by the following formula. The yield was 92%. The above crude product was found by chiral gas chromatography to have a conversion of 100% and an enantiomeric excess of 100 %ee. In terms of severing position selectivity whether the N-C* bond is severed at the broken line "a" to produce a compound A or at the broken line "b" to produce a compound B (i.e., the above optically active (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethylamine N-monomethyl) in the above formula 11, the above crude product was found by chiral gas chromatography to contain 1 part by mole of the compound A and 99 parts by mole of the compound B. 1H-NMR(TMS, CDCl3), δ ppm: 1.38 (d, 6.4 Hz, 3H), 1.45 (br, 1H), 2.30 (s,3H), 3.81 (q, 6.4 Hz, 1H), 7.75 (Ar-H, 1H), 7.80 (Ar-H, 2H)
References:
WO2004/22521,2004,A1 Location in patent:Page 57-58
![Benzenemethanamine, N,α-dimethyl-N-[(1R)-1-phenylethyl]-3,5-bis(trifluoromethyl)-, (αR)-](/CAS/20210305/GIF/672906-99-9.gif)
672906-99-9
0 suppliers
inquiry
![(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/334477-60-0.gif)
334477-60-0
39 suppliers
$95.00/250mg
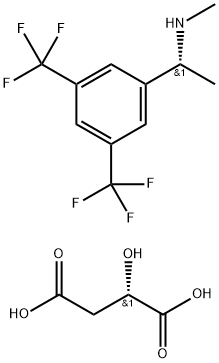
935534-56-8
23 suppliers
$333.00/1g
![(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/334477-60-0.gif)
334477-60-0
39 suppliers
$95.00/250mg
![Methanamine, N-[1-[3,5-bis(trifluoromethyl)phenyl]ethylidene]-](/CAS/20210305/GIF/1333317-69-3.gif)
1333317-69-3
0 suppliers
inquiry
![(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/334477-60-0.gif)
334477-60-0
39 suppliers
$95.00/250mg
![(S)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/511256-36-3.gif)
511256-36-3
37 suppliers
$168.00/1g
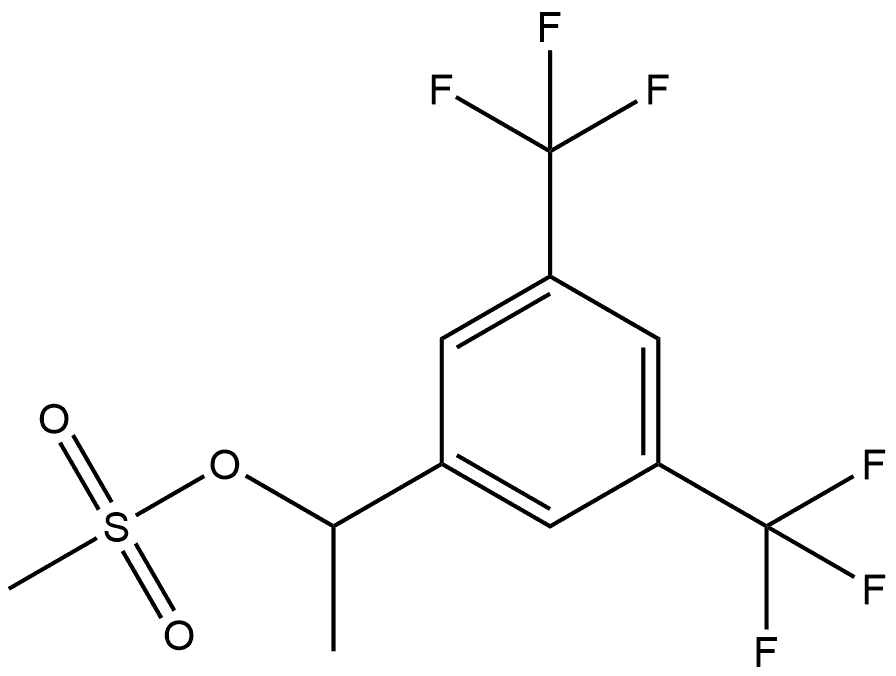
737786-85-5
0 suppliers
inquiry

74-89-5
0 suppliers
$13.44/25ML
![(R)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/334477-60-0.gif)
334477-60-0
39 suppliers
$95.00/250mg
![(S)-N-Methyl-1-[3,5-bis(trifluoromethyl)phenyl]ethylamine](/CAS/GIF/511256-36-3.gif)
511256-36-3
37 suppliers
$168.00/1g