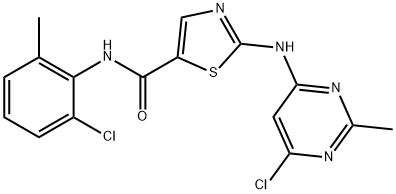
N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide synthesis
- Product Name:N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide
- CAS Number:302964-08-5
- Molecular formula:C16H13Cl2N5OS
- Molecular Weight:394.28
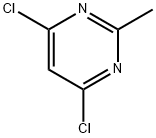
1780-26-3
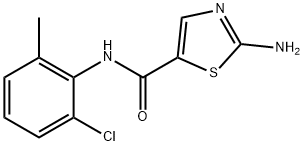
302964-24-5
![N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide](/CAS/GIF/302964-08-5.gif)
302964-08-5
General procedure for the synthesis of N-(2-chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide from 4,6-dichloro-2-methylpyrimidine and 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide: To 80 mL of tetrahydrofuran, cooled to -25 °C, was added 8.73 g of potassium hydride (30 wt%, 65.38 mmol) and stirred for 10 minutes; 5.00 g of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide was slowly added and the temperature was maintained at -25 °C; followed by the addition of 3.65 g of 4,6-dichloro-2-methylpyrimidine to a tetrahydrofuran solution (7 mL), the temperature was maintained stable and the reaction mixture was stirred for 4 hours at -10 °C. After completion of the reaction, 1 M hydrochloric acid was slowly added to quench the reaction, pH was adjusted to 6, the temperature was controlled at 0-5 °C for 2 h. Crystallization was carried out by centrifugation and washed with tetrahydrofuran. The crude product was obtained as 7.27 g in 98.7% yield and 99.95% purity (HPLC) with a maximum single heterogeneous content of 0.03%.

1780-26-3
476 suppliers
$10.00/10g

302964-24-5
408 suppliers
$9.00/250mg
![N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide](/CAS/GIF/302964-08-5.gif)
302964-08-5
297 suppliers
$7.00/250mg
Yield:302964-08-5 98.7%
Reaction Conditions:
with potassium hydride in tetrahydrofuran at -25 - -10; for 4 h;Reagent/catalyst;Temperature;
Steps:
1
To 80 ml of tetrahydrofuran cooled to -25 ° C, 8.73 g of potassium hydride (30% by weight, 65.38 mmol) was added; stirred and stirred for 10 min; slowly added to 5.00 g of 2-amino-N- (2-chloro- Phenyl) thiazole-5-carboxamide was added at a temperature of -25 ° C and a solution of 3.65 g of tetrahydrofuran (7 ml) containing 2-methyl-4,6-dichloropyrimidine was added in advance to a solution of In the reaction system, the temperature was stabilized and the reaction was stirred at -10 ° C for 4 hours. Then, ImoVL hydrochloric acid quenched reaction was slowly added. The pH was adjusted to 6, controlled at 0-5 ° C, crystallized for 2 h, centrifuged, washed with THF, The crude product was 7.27g. The yield was 98.7%, the purity was 99.95% (HPLC), and the maximum single hetero content was 0.03%
References:
CN104788445,2017,B Location in patent:Paragraph 0016; 0026; 0027; 0029; 0031; 0033; 0035; 0037

77296-31-2
2 suppliers
inquiry
![N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide](/CAS/GIF/302964-08-5.gif)
302964-08-5
297 suppliers
$7.00/250mg
834888-64-1
3 suppliers
inquiry
![N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide](/CAS/GIF/302964-08-5.gif)
302964-08-5
297 suppliers
$7.00/250mg
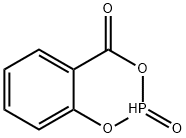
80337-06-0
0 suppliers
inquiry
![N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide](/CAS/GIF/302964-08-5.gif)
302964-08-5
297 suppliers
$7.00/250mg
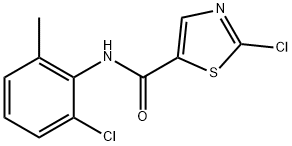
302964-11-0
12 suppliers
inquiry
![N-(2-Chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide](/CAS/GIF/302964-08-5.gif)
302964-08-5
297 suppliers
$7.00/250mg