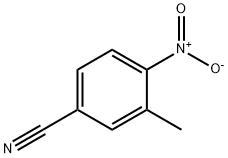
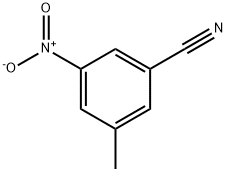
3-METHYL-4-NITROBENZONITRILE synthesis
- Product Name:3-METHYL-4-NITROBENZONITRILE
- CAS Number:96784-54-2
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
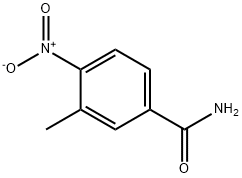
99584-85-7

96784-54-2
General procedure for the synthesis of 3-methyl-4-nitrobenzonitrile from 3-methyl-4-nitrobenzamide: 3-methyl-4-nitrobenzamide (5 g, 28 mmol) was dissolved in 300 ml of toluene under stirring conditions, followed by the slow addition of phosphorus oxychloride (POCl3, 22 g, 140 mmol). After the addition was completed, the reaction mixture was heated to reflux for 24 hours. Upon completion of the reaction, the mixture was carefully poured into ice water and extracted three times with ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Finally, the residue was purified by ethanol recrystallization to afford the target product 3-methyl-4-nitrobenzonitrile (3.2 g, 70% yield).
3113-71-1
491 suppliers
$6.00/25g
70-55-3
594 suppliers
$6.00/100g

96784-54-2
123 suppliers
$7.00/250mg
Yield: 35.9 g (23 %)
Reaction Conditions:
with pyridine;phosphorus pentachloride;trichlorophosphate in dichloromethane
Steps:
1.A Part A
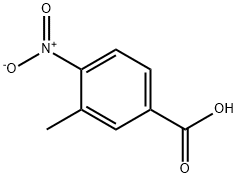
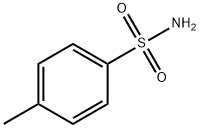
Part A Preparation of the intermediate 4-nitro-3-tolunitrile, represented by the formula: A mixture of PCl5 (200 g, 0.96 mole) and p-toluene sulfonamide (90 g, 0.52 mole) was treated with 3-methyl-4-nitrobenzoic acid (82 g, 0.44 mole). The reaction was stirred at room temperature for 1 hour at which time it was heated to 140°C and the POCl3 (150 ml) distilled from the mixture. The reaction was cooled to 5°C, pyridine (200 ml) was carefully added and the mixture allowed to stand overnight. The reaction was carefully diluted to 1 L with H2O and the mixture stirred for 3 hours. The brown solid was filtered, washed with H2O and triturated with 5 N aqueous NaOH. The remaining solid was filtered, washed with H2O and dried to afford 60.0 g of a powder. The solid was stirred in 1200 ml CH2Cl2 and filtered. The supernatant was evaporated in vacuo and the residue recrystallized from EtOH to give 35.9 g (23 %) of 4-nitro-3-tolunitrile. MS(FD), m/e 162 (M+).
References:
ELI LILLY AND COMPANY EP655439, 1995, A2

99584-85-7
60 suppliers
$50.39/1g

96784-54-2
123 suppliers
$7.00/250mg

3113-71-1
491 suppliers
$6.00/25g

96784-54-2
123 suppliers
$7.00/250mg

35675-46-8
24 suppliers
inquiry

96784-54-2
123 suppliers
$7.00/250mg
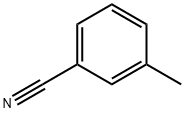
620-22-4
227 suppliers
$11.00/25mL

96784-54-2
123 suppliers
$7.00/250mg

64113-86-6
70 suppliers
inquiry
124289-22-1
75 suppliers
inquiry