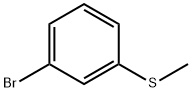
3-Bromothioanisole synthesis
- Product Name:3-Bromothioanisole
- CAS Number:33733-73-2
- Molecular formula:C7H7BrS
- Molecular Weight:203.1

624-92-0
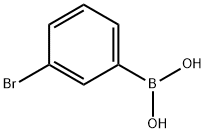
89598-96-9

33733-73-2
GENERAL METHOD: 3-bromophenylboronic acid (1.0 mmol), dimethyl disulfide (2.0 mmol), di-tert-butyl peroxide (DTBP, 3.0 mmol) and acetonitrile (CH3CN, 2.0 mL) were added sequentially to a sealed tube. The reaction mixture was stirred at 120 °C in air for 12 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was diluted with deionized water (5 mL) and subsequently extracted with ethyl acetate (EtOAc, 4 × 10 mL). The organic phases were combined, washed with saturated saline (3 × 10 mL), dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated. The crude product was purified by silica gel column chromatography with ethyl acetate/hexane as eluent (v/v = 1:30 to 1:100). The resulting 3-bromothioanisole was characterized by spectroscopic analyses (NMR hydrogen spectroscopy, carbon spectroscopy, etc.) and compared with the data of known compounds (see Supporting Information for details).

624-92-0
351 suppliers
$20.62/5 mL
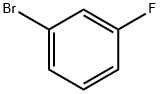
1073-06-9
447 suppliers
$6.00/10g

33733-73-2
165 suppliers
$5.00/1g
Yield: 80%
Reaction Conditions:
with potassium tert-butylate in acetonitrile at 50; for 6 h;Sealed tube;
Steps:
4.2.1. Synthetic procedures
General procedure: Aryl fluorides (1.0 mmol), CH3SSCH3 (1.2 mmol), t-BuOK (3.0 mmol) and CH3CN (2 mL) were taken in a 25-ml sealed tube. The reaction mixture was stirred at 50 C for 6 h under air. After cooling to room temperature, the product was diluted with H2O (5 mL) and extracted with EtOAc (4 × 10 mL). The extracts were combined and washed by brine (3 × 10 mL), dried over MgSO4, filtered, and evaporated, and purified by chromatography on silica gel to obtain the desired products with ethyl acetate/hexane (v/v = 1:301:100). The products were characterized by their spectral and analytical data and compared with those of the known compounds (See supporting information).
References:
Huang, Dayun;Wu, Xiangmei [Journal of Fluorine Chemistry,2021,vol. 245,art. no. 109778]

624-92-0
351 suppliers
$20.62/5 mL
591-18-4
398 suppliers
$5.00/5g

33733-73-2
165 suppliers
$5.00/1g

624-92-0
351 suppliers
$20.62/5 mL

89598-96-9
349 suppliers
$9.00/1g

33733-73-2
165 suppliers
$5.00/1g

624-92-0
351 suppliers
$20.62/5 mL
108-36-1
460 suppliers
$10.00/1g

33733-73-2
165 suppliers
$5.00/1g

