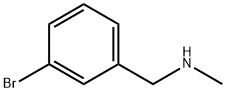
(3-BROMOBENZYL)METHYLAMINE synthesis
- Product Name:(3-BROMOBENZYL)METHYLAMINE
- CAS Number:67344-77-8
- Molecular formula:C8H10BrN
- Molecular Weight:200.08
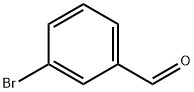
3132-99-8

74-89-5

67344-77-8
Step B: At room temperature, 3-bromobenzaldehyde (47.5 mL, 0.4 mol) was dissolved in methanol (460 mL), followed by the addition of an aqueous solution of methylamine (35 mL, 0.4 mol, 40 wt% solution). The reaction mixture was cooled to 0 °C and sodium borohydride (22 g, 0.6 mol) was added in batches. The reaction mixture was kept stirred at 0 °C for 3.5 h and then gradually warmed up to room temperature. After completion of the reaction, the mixture was concentrated under reduced pressure and subsequently partitioned with dichloromethane and water. The aqueous layer was separated and washed with dichloromethane (3×). The organic phases were combined and washed sequentially with saturated sodium bicarbonate solution and brine, then dried over anhydrous magnesium sulfate. After filtration, the organic phase was concentrated under reduced pressure to afford the target product N-methyl-3-bromobenzylamine (76 g, 81%) as a clear oil. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.48 (d, J = 1.5 Hz, 1H), 7.40-7.37 (m, 1H), 7.24-7.16 (m, 2H), 3.73 (s, 2H), 2.45 (s, 3H).
Yield: 81%
Reaction Conditions:
with sodium tetrahydroborate in methanol;water at 0 - 20; for 3.5 h;Product distribution / selectivity;
Steps:
72.B; 81.A
Step B: To a solution of 3-bromobenzaldehyde (47.5 mL, 0.4 mol) in methanol (460 mL) at room temperature was added a solution of methylamine in water (35 mL, 0.4 mol, 40 wt. % solution). The resultant solution was cooled to 0° C. and sodium borohydride (22 g, 0.6 mol) was added to it in portions. The reaction solution was stirred at 0° C. for 3 hours and 30 minutes, and then warmed to room temperature. The resultant reaction mixture was concentrated in vacuo and partitioned between dichloromethane and water. The aqueous layer was separated and washed with dichloromethane (3×). The combined organic extract was washed with saturated sodium bicarbonate and brine, dried over magnesium sulfate, filtered and concentrated in vacuo to give the desired benzylamine (76 g, 81%) as a clear oil: 1H NMR (300 MHz, CDCl3) δ 7.48 (d, J=1.5 Hz, 1H), 7.40-7.37 (m, 1H), 7.24-7.16 (m, 2H), 3.73 (s, 2H), 2.45 (s, 3H).; Example 81 Preparation of (+)-N-methyl-2-(2-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[c]azepin-8-yl)ethanesulfonamide, L-tartrate salt and (-)-N-methyl-2-(2-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[c]azepin-8-yl)ethanesulfonamide, L-tartrate salt Step A: To a solution of 3-bromobenzaldehyde (47.5 mL, 0.4 mol) in methanol (460 mL) at room temperature was added a solution of methylamine in water (35 mL, 0.4 mol, 40 wt % solution). The resultant solution was cooled to 0° C. and sodium borohydride (22 g, 0.60 mol) was added to it in portions. The reaction solution was stirred at 0° C. for 3 hours and 30 minutes, and then warmed to room temperature. The resultant reaction mixture was concentrated in vacuo and partitioned between dichloromethane and water. The aqueous layer was separated and washed with dichloromethane (3×). The combined organic extracts were washed with saturated sodium bicarbonate and brine, dried over magnesium sulfate, filtered and concentrated in vacuo to give the benzylamine (76 g, 81%) as a clear oil: 1H NMR (300 MHz, CDCl3) δ 7.48 (d, J=1.5 Hz, 1H), 7.40-7.37 (m, 1H), 7.24-7.16 (m, 2H), 3.73 (s, 2H), 2.45 (s, 3H).
References:
AMR Technology, Inc. US2007/21408, 2007, A1 Location in patent:Page/Page column 94; 103
24424-99-5
866 suppliers
$13.50/25G
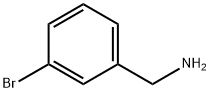
10269-01-9
204 suppliers
$8.00/1g

67344-77-8
123 suppliers
$9.00/1g
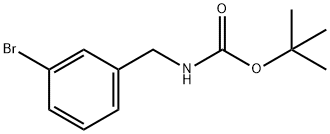
171663-13-1
75 suppliers
$30.00/1g

67344-77-8
123 suppliers
$9.00/1g
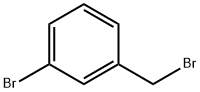
823-78-9
281 suppliers
$8.00/10g

67344-77-8
123 suppliers
$9.00/1g