
3-BROMO-4-METHOXY-BENZENESULFONYL CHLORIDE synthesis
- Product Name:3-BROMO-4-METHOXY-BENZENESULFONYL CHLORIDE
- CAS Number:23094-96-4
- Molecular formula:C7H6BrClO3S
- Molecular Weight:285.54
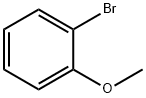
578-57-4

23094-96-4
General procedure for the synthesis of 3-bromo-4-methoxybenzene-1-sulfonyl chloride from 2-bromoanisole: 2-bromo-1-methoxybenzene (1.87 g, 10.0 mmol) was dissolved in chloroform (5 mL), and the solution was cooled in an iced salt bath to -5 °C to 0 °C. Chlorosulfonic acid (2.0 mL, 30.0 mmol) was slowly added dropwise over 30 minutes, and after completion of the dropwise addition, the reaction mixture was gradually warmed to room temperature over 1 hour. Subsequently, the reaction mixture was poured onto crushed ice and transferred to a dispensing funnel. The aqueous layer was separated and extracted twice with chloroform. The organic layers were combined, dried and concentrated to give 3-bromo-4-methoxybenzenesulfonyl chloride (2.80 g, 98%). 3-Bromo-4-methoxybenzenesulfonyl chloride (2.80 g, 9.8 mmol) was dissolved in dichloromethane (30 mL), triethylamine (1.76 mL, 12.6 mmol) was added to the solution, followed by slow dropwise addition of tert-butylamine (1.33 mL, 12.6 mmol). The reaction mixture was allowed to stand for 2 hours and then poured into a mixture of 5% citric acid solution and dichloromethane. The organic layer was separated and the aqueous layer was extracted twice with dichloromethane. The organic layers were combined, dried and concentrated. The crude product was recrystallized by ethyl acetate/hexane mixed solvent to give 3-bromo-N-tert-butyl-4-methoxybenzenesulfonamide (2.43 g, 77%).

578-57-4
307 suppliers
$6.00/10g

23094-96-4
62 suppliers
$10.00/100mg
Yield: 98%
Reaction Conditions:
with chlorosulfonic acid in dichloromethane at -5 - 20; for 1.5 h;
Steps:
6 Reference 6; 3-BROMO-N-TERT-BUTYL-4-METHOXYBENZENESULFONAMIDE
L-BROMO-2-METHOXY-BENZENE (1.87 g, 10.0 mmol) was dissolved in chloroform (5 mL) and the solution was cooled in an ice-salt bath to-5° C to 0° C. The cooled solution was carefully charged with CHLOROSULFONIC acid (2.0 mL, 30.0 mmol) over 30 minutes and the mixture was allowed to warm to room temperature over 1 hour. The mixture then was poured onto chopped ice and transferred to a separatory funnel. The aqueous layer was separated and extracted (X2). The combined organic layers were dried and concentrated to give 3-bromo-4-methoxyphenylsulfonyl chloride (2.80 g, 98%). 3-Bromo-4-methoxyphenylsulfonyl chloride (2.80 g, 9.8 mmol) was dissolved in dichloromethane (30 mL) and the solution was treated with triethylamine (1.76 mL, 12.6 mmol), followed by the dropwise addition of t-butylamine (1.33 mL, 12.6 mmol). The mixture was allowed to stand for 2 hours and then was poured onto a mixture of 5% citric acid solution and dichloromethane. The organic layer was separated and the aqueous layer was extracted with dichloromethane/ (X2). The combined organic layers were dried and concentrated. Crystallization of the crude solid from ethyl acetate/hexane gave 3-BROMO-N- tert-butyl-4-methoxybenzenesulfonamide (2.43 g, 77%)
References:
AXYS PHARMACEUTICALS, INC. WO2004/50637, 2004, A2 Location in patent:Page 68-69