
3-BENZYLOXYBROMOBENZENE synthesis
- Product Name:3-BENZYLOXYBROMOBENZENE
- CAS Number:53087-13-1
- Molecular formula:C13H11BrO
- Molecular Weight:263.13
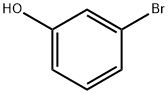
591-20-8
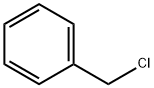
100-44-7

53087-13-1
1. Anhydrous potassium carbonate (37.32 g, 270 mmol) and benzyl chloride (11.50 mL, 99 mmol) were added to a suspension of anhydrous ethanol (300 mL) containing m-bromophenol (15.57 g, 90 mmol) under nitrogen protection. The reaction mixture was heated to reflux for 4 hours. After completion of the reaction, the ethanol was removed by vacuum filtration and evaporation. The residue was extracted with ethyl acetate (EtOAc) and the combined organic layers were washed sequentially with water, 2 M NaOH solution, saturated brine and 2 M HCl solution, dried over anhydrous sodium sulfate (Na2SO4) and concentrated to give the yellow solid product 3-benzyloxybromobenzene (22.02 g, 93%). 2. To a 250 mL two-necked round-bottomed flask, protected by nitrogen, were added dry magnesium chips (2.18 g, 90 mmol) and trace iodine, equipped with two rubber septa and stirred. Anhydrous tetrahydrofuran (THF, 40 mL) solution of 3-benzyloxybromobenzene (15.79 g, 60 mmol) was slowly added through a syringe and kept at reflux. After addition, stirring was continued at reflux for 5 hours. After the reaction mixture was cooled to -30°C, anhydrous THF (60 mL) solution of trimethyl borate (9.36 g, 90 mmol) was slowly added. After addition, the mixture was stirred at room temperature overnight, followed by treatment with saturated ammonium chloride (NH4Cl) solution and continued stirring at room temperature for 1 hour. THF was removed by rotary evaporation, the aqueous layer was extracted with EtOAc, dried over anhydrous Na2SO4, filtered and concentrated to give a yellow crude product. The crude product was purified by recrystallization in water to give white solid target product (7.53 g, 55%).

591-20-8
352 suppliers
$10.00/1g

100-39-0
433 suppliers
$10.00/10g

53087-13-1
218 suppliers
$11.00/5g
Yield:53087-13-1 100%
Reaction Conditions:
with potassium carbonate in acetone; for 15 h;Reflux;
Steps:
79.79-1 Preparation of 1-(benzyloxy)-3-bromobenzene
A solution of 3-bromophenol (3 g, 17.34 mmol) in acetone (100 mL) was added with benzyl bromide (2.2 mL, 19.07 mmol) and K2CO3 (7.0 g, 52.02 mmol), and the resulting mixture was refluxed for 15 hours. The reaction mixture was cooled to room temperature, filtered, and washed with acetone. The filtrate was concentrated and purified by silica gel chromatography to obtain the title compound (white solid, 5.1 g, 100% yield). 1H NMR (300 MHz, CDCl3) δ 7.50 - 7.30 (m, 5H), 7.20 - 7.01 (m, 3H), 6.98 - 6.80 (m, 1H), 5.05 (s, 2H).
References:
EP2963027,2016,A1 Location in patent:Paragraph 1227; 1228; 1229

591-20-8
352 suppliers
$10.00/1g

100-44-7
658 suppliers
$13.50/250G

53087-13-1
218 suppliers
$11.00/5g
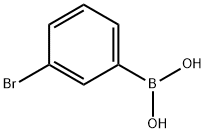
89598-96-9
346 suppliers
$9.00/1g

100-39-0
433 suppliers
$10.00/10g

53087-13-1
218 suppliers
$11.00/5g
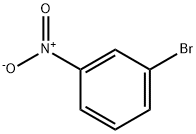
585-79-5
21 suppliers
$15.00/1g
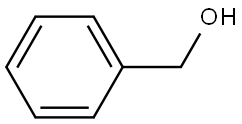
100-51-6
1440 suppliers
$5.00/100g

53087-13-1
218 suppliers
$11.00/5g

591-20-8
352 suppliers
$10.00/1g
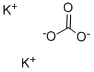
584-08-7
1242 suppliers
$5.00/100g

100-44-7
658 suppliers
$13.50/250G

53087-13-1
218 suppliers
$11.00/5g