
3-Amino-2-methylphenylboronic acid, pinacol ester synthesis
- Product Name:3-Amino-2-methylphenylboronic acid, pinacol ester
- CAS Number:882678-96-8
- Molecular formula:C13H20BNO2
- Molecular Weight:233.11

910235-64-2

882678-96-8
General procedure for the synthesis of 2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline from 4,4,5,5-tetramethyl-2-(2-methyl-3-nitrophenyl)-1,3,2-dioxaborolan-2-yl)aniline: in a 500 mL round-bottomed flask 4,4,5,5-tetramethyl-2-(2-methyl-3-nitrophenyl) -1,3,2-dioxaborolane(6) (8.44 g, 32.1 mmol) and methanol (150 mL) in a 500 mL round-bottom flask equipped with a magnetic stirrer. After the reaction system was evacuated twice and replaced with argon, 10% palladium carbon catalyst (50% wet weight, 425 mg dry weight) was added. The reaction system was again evacuated and replaced with hydrogen three times. The reaction was stirred at room temperature for 13 hours under hydrogen balloon pressure. Upon completion of the reaction, the system was evacuated twice and replaced with argon, filtered through a diatomaceous earth pad (521) and the filtrate was concentrated under vacuum. The resulting residue was dried under high vacuum for 24 h to afford 2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (7) in quantitative yield (8.16 g) as a white solid with a melting point of 110-112 °C; mass spectrum (ESI+) m/z 234 (M+H).

910235-64-2
37 suppliers
$108.00/1g

882678-96-8
79 suppliers
$22.00/100mg
Yield: 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 20; for 13 h;
Steps:
1
2-Methyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine (7); A 500-mL round-bottomed flask equipped with a magnetic stirrer was charged with 4,4,5,5-Tetramethyl-2-(2-methyl-3-nitro-phenyl)-[1,3,2]dioxaborolane (6) (8.44 g; 32.1 mmol) and methanol (150 mL). The reaction flask was twice evacuated and back-filled with argon. 10% Palladium on charcoal (50% wet, 425 mg dry weight) was then added to the solution, and the reaction flask evacuated and back-filled with hydrogen three times. The reaction was then stirred under balloon pressure of hydrogen at room temperature for 13 h. After this time, the flask was twice evacuated and back-filled with argon, then filtered through a pad of Celite 521 and the filtrate concentrated in vacuo. The resulting residue was dried under high vacuum for 1 d to afford a quantitative yield (8.16 g) of 2-methyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine (7) as a white solid: mp 110-112° C.; MS (ESI+) m/z 234 (M+H).
References:
Zhao, Zhongdong;Zhichkin, Pavel E.;Stafford, Douglas G.;Kropf, Jeffrey E.;BLOMGREN, Peter A.;Currie, Kevin S.;Lee, Seung H.;Mitchell, Scott A.;Xu, Jianjun;Schmitt, Aaron C. US2009/82330, 2009, A1 Location in patent:Page/Page column 24
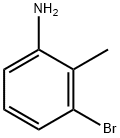
55289-36-6
295 suppliers
$5.00/1g
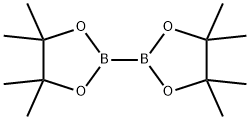
73183-34-3
585 suppliers
$6.00/5g

882678-96-8
79 suppliers
$22.00/100mg
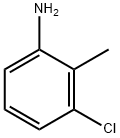
87-60-5
443 suppliers
$8.00/25g

73183-34-3
585 suppliers
$6.00/5g

882678-96-8
79 suppliers
$22.00/100mg
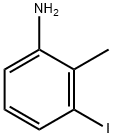
172681-47-9
82 suppliers
$39.00/1g

73183-34-3
585 suppliers
$6.00/5g

882678-96-8
79 suppliers
$22.00/100mg

55289-36-6
295 suppliers
$5.00/1g
25015-63-8
215 suppliers
$22.39/5G

882678-96-8
79 suppliers
$22.00/100mg