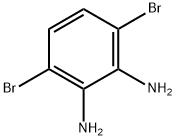
3,6-dibroMo-1,2-BenzenediaMine synthesis
- Product Name:3,6-dibroMo-1,2-BenzenediaMine
- CAS Number:69272-50-0
- Molecular formula:C6H6Br2N2
- Molecular Weight:265.93
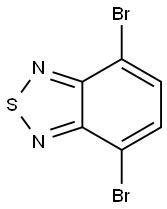
15155-41-6

69272-50-0
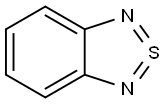
GENERAL STEPS: To a suspension of 4,7-dibromo-2,1,3-benzothiadiazole (1) (5.88 g, 20 mmol) in ethanol (190 mL) was added sodium borohydride (14 g, 0.37 mol) in batches at 0 °C. The reaction mixture was stirred at room temperature for 20 hours. Upon completion of the reaction, the volatile solvent was evaporated and water (200 mL) was added, followed by extraction with dichloromethane (3 x 30 mL). The organic phases were combined, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to afford 3,6-dibromo-1,2-benzenediamine (2) (4.7 g, 87% yield) as a light yellow solid. Intermediate 2 was used directly in the next reaction without further purification. Selenium dioxide (1.17 g, 10.5 mmol) was dissolved in hot water (22 mL) and this solution was added to a refluxing ethanol (55 mL) solution of intermediate 2 (2.7 g, 10 mmol). The reaction mixture was heated to reflux for 2 hours. After cooling to room temperature, the precipitate was collected by filtration and recrystallized from ethyl acetate to give 4,7-dibromo-2,1,3-benzoselenadiazole (3) (2.8 g, 80% yield) as golden-yellow needle-like crystals.1H NMR (400 MHz, CDCl3) δ (ppm): 7.65 (s, 2H). Melting point 285-287°C. 13C NMR (100 MHz, CDCl3) δ (ppm): 157.2, 132.1, 116.5. Elemental analysis (C6H2Br2N2Se) Calculated value: C, 21.14; H, 0.59; N, 8.22. Measured value: C, 21.34; H, 0.92; N, 7.98.

15155-41-6
326 suppliers
$21.00/1g
30757-50-7
130 suppliers
$60.00/100mg
Yield:30757-50-7 98%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 0 - 20; for 20 h;
Steps:
Preparation of compound 1.
Sodium borohydride (4.2 g, 110 mmol) was added portion wise into a solution of 4,7-Dibromo-2,1,3-Benzothiadiazole (3.3 g, 11.2 mmol) in ethanol (100 mL) at 0 °C. The resulting mixture was stirred at room temperature for 20 h. The reaction mixture was quenched by adding 50 mL of water, and extracted with CH2Cl2 (40 mL 2). The combined organic layers were washed with water and brine successively, dried over anhydrous sodium sulfate. Removing the solvent under rotary evaporator, the crude product was purified by column chromatography using hexane/ethyl acetate (5/1, v/v) as an eluent to afford compound 1 as an white solid (2.9 g, yield: 98%), mp: 128.2-131.3 °C. 1H NMR (600 MHz, CDCl3) δ: 6.84 (s, 2H), 3.89 (s, 4H). 13C NMR (150 MHz, CDCl3) δ: 133.7, 123.3, 109.7. Anal. calcd for C6H6Br2N2: C, 27.10; H, 2.27; N, 10.53; found: C, 27.04; H, 2.28; N, 10.49.
References:
Chen, Hongjin;Han, Yiying;Liu, Jian;Wang, Wenyuan;Zhu, Jianjun [Polymer,2022,vol. 240,art. no. 124485]
22706-22-5
12 suppliers
inquiry

15155-41-6
326 suppliers
$21.00/1g

30757-50-7
130 suppliers
$60.00/100mg

22706-22-5
12 suppliers
inquiry

30757-50-7
130 suppliers
$60.00/100mg
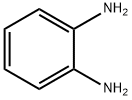
95-54-5
542 suppliers
$9.00/1g

30757-50-7
130 suppliers
$60.00/100mg
480-96-6
202 suppliers
$10.00/1g

30757-50-7
130 suppliers
$60.00/100mg