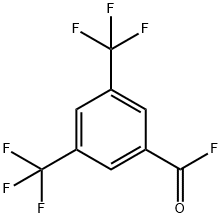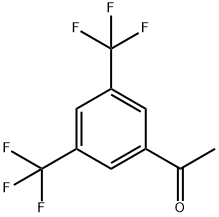
3',5'-Bis(trifluoromethyl)acetophenone synthesis
- Product Name:3',5'-Bis(trifluoromethyl)acetophenone
- CAS Number:30071-93-3
- Molecular formula:C10H6F6O
- Molecular Weight:256.14
![(R)-[3,5-bis(trifluoromethyl)phenyl]ethanol](/CAS/20180703/GIF/68120-60-5.gif)
68120-60-5

30071-93-3
General procedure for the synthesis of 3,5-bis(trifluoromethyl)acetophenone from (R)-1-[3,5-bis(trifluoromethyl)-phenyl]ethanol: BTC (0.41 g, 1.39 mmol) was dissolved in anhydrous dichloromethane (5 mL) under the protection of nitrogen and cooled to -15 °C in an ice-salt bath. At this temperature, a solution of (R)-1-[3,5-bis(trifluoromethyl)-phenyl]ethanol (1.24 g, 4.17 mmol) in anhydrous dichloromethane (5 mL) was slowly added dropwise for 0.5 hours. After continued stirring for 0.5 h, an anhydrous dichloromethane (5 mL) solution of benzyl alcohol (0.3 g, 2.78 mmol) was added dropwise for 0.5 h at the same temperature. After stirring for 0.5 h, triethylamine (0.84 g, 8.34 mmol) was slowly added while making sure that the reaction temperature was kept below -15 °C. Upon completion of the reaction, 10% aqueous hydrochloric acid was added dropwise under ice bath conditions until the pH of the reaction mixture reached 2. The organic layer was separated by extracting the mixture with hexane or petroleum ether (10 mL x 2). After concentration of the organic layer, it was purified by fast chromatography (silica gel; hexane) to afford the target product 3,5-bis(trifluoromethyl)acetophenone (0.27 g, 92% yield). The aqueous layer can be used to recover by-product V and excess (R)-1-[3,5-bis(trifluoromethyl)-phenyl]ethanol.
328-70-1
439 suppliers
$10.00/25 g

30071-93-3
389 suppliers
$10.00/10 g
Yield:30071-93-3 82%
Reaction Conditions:
with acetic anhydride;magnesium in tetrahydrofuran;water
Steps:
3 1-(3,5-Bis(trifluoromethyl)phenyl)ethan-1-one
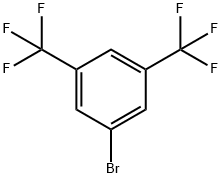
EXAMPLE 3 1-(3,5-Bis(trifluoromethyl)phenyl)ethan-1-one A solution of 3,5-Bis(trifluoromethyl)bromobenzene (29.3 g) in 30 mL of THF was added to a mixture of magnesium granules (5.10 g) in THF (200 mL) heated at reflux (the reaction was initiated with approximately 5 mL of the bromide solution; the remainder was added slowly over 1 h). The mixture was aged for 30 min at reflux, cooled to RT and added over 1 h to a solution of acetic anhydride (40 mL) in THF (40 mL) maintained at -15° C. The resulting dark brown mixture was warmed to 10° C. in a water bath, and water (300 mL) was added. The pH of the vigorously stirred biphasic mixture was adjusted to 8.0 using 50% NaOH. MTBE (300 mL) was added, the layers were separated and the aqueous layer was further extracted with MTBE (3*150 mL). The organic layers were combined and concentrated in vacuo (bath at 30-35° C.; 50-80 torr). The concentrate was then distilled at atmospheric pressure to provide the pure product (20.7 g; 82% yield) with a boiling point of 187-189° C.
References:
McNamara, James M.;Zhao, Matthew M. US2002/22725, 2002, A1 Brands, Karel M. Jos;Tsay, Fuh-Rong;Conrad, Karen M.;Zhao, Matthew M. US2002/52494, 2002, A1

328-70-1
439 suppliers
$10.00/25 g
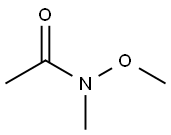
78191-00-1
291 suppliers
$6.00/5g

30071-93-3
389 suppliers
$10.00/10 g

328-70-1
439 suppliers
$10.00/25 g
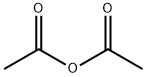
108-24-7
0 suppliers
$14.00/250ML

30071-93-3
389 suppliers
$10.00/10 g

328-70-1
439 suppliers
$10.00/25 g
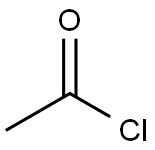
75-36-5
592 suppliers
$17.92/100G

30071-93-3
389 suppliers
$10.00/10 g