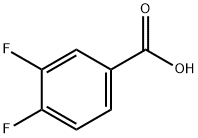
3,4-Difluorobenzoic acid synthesis
- Product Name:3,4-Difluorobenzoic acid
- CAS Number:455-86-7
- Molecular formula:C7H4F2O2
- Molecular Weight:158.1
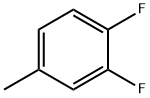
2927-34-6

455-86-7
Example 5: Synthesis of 3,4-difluorobenzoic acid. In a 100 mL jacketed reactor, 200 mL of acetic acid, 0.05 g of manganese(II) acetate, 1.91 g of sulfuric acid, and 14.1 g of 3,4-difluorotoluene were added sequentially. The reaction mixture was cooled to 16°C, followed by the slow passage of 4.0 g of ozone over 60 min. Upon completion of the ozonolysis reaction, the reaction system was purged with nitrogen to remove residual ozone. HPLC or GC analysis of the reaction mixture showed that the residue of 3,4-difluorotoluene was <0.1%; the yield of 3,4-difluorobenzoic acid was 98%.

2927-34-6
206 suppliers
$6.00/1g

455-86-7
420 suppliers
$5.00/10g
Yield:455-86-7 98%
Reaction Conditions:
with sulfuric acid;ozone;acetic acid;manganese(II) acetate at 16; for 1 h;
Steps:
5 Example 5:
Example 5: 3,4-Difluorobenzoic Acid In a 100 ml jacketed vessel, 200 ml of acetic acid, 0.05 g of manganese(II) acetate, 1.91 g of sulfuric acid and 14.1 g of 3,4-difluorotoluene were initially charged. The mixture was cooled to 16° C. and 4.0 g of ozone were introduced within a period of 60 minutes. After completion of ozonolysis, the ozone present in the solvent was blown out using nitrogen. Analysis of the reaction mixture by means of HPLC or GC gave the following results: 3,4-difluorotoluene: >0.1%; 3,4-difluorobenzoic acid: 98%
References:
US2003/216577,2003,A1 Location in patent:Page/Page column 4
6998-83-0
20 suppliers
$704.00/1G

455-86-7
420 suppliers
$5.00/10g
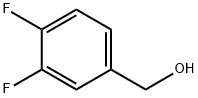
85118-05-4
196 suppliers
$9.00/5g

455-86-7
420 suppliers
$5.00/10g

34036-07-2
366 suppliers
$7.00/5g
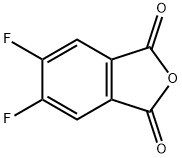
18959-30-3
196 suppliers
$6.00/100mg

455-86-7
420 suppliers
$5.00/10g
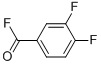
127269-25-4
2 suppliers
inquiry

455-86-7
420 suppliers
$5.00/10g