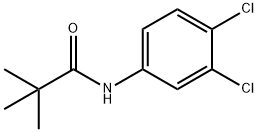
3',4'-DICHLOROPIVALANILIDE synthesis
- Product Name:3',4'-DICHLOROPIVALANILIDE
- CAS Number:7160-22-7
- Molecular formula:C11H13Cl2NO
- Molecular Weight:246.13
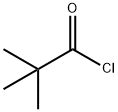
3282-30-2
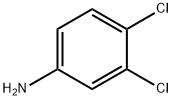
95-76-1

7160-22-7
The general procedure for the synthesis of 3-chloro-2-(chloromethyl)-2-methyl-N-phenylpropionamide from pivaloyl chloride and 3,4-dichloroaniline was carried out as follows: firstly, the preparation of N-(3,4-dichlorophenyl)-2,2-dimethylpropionamide was carried out. 3,4-Dichloroaniline (150 g) was dissolved in tert-butyl methyl ether (TBME, 1 L) and the solution was cooled to 10-15 °C. Under vigorous stirring, 30% aqueous sodium hydroxide solution (141 g, 1.14 eq.) was slowly added, followed by dropwise addition of pivaloyl chloride (PivCl, 126 mL) at a rate that controlled the internal temperature to not exceed 30°C. During the dropwise addition, the reaction mixture gradually thickened and precipitated a white solid product. After completion of the dropwise addition (about 10-15 minutes), the reaction mixture was heated to 30-35°C and maintained for 1 hour, followed by cooling to room temperature. The mixture was placed at -5°C overnight and then filtered. The filter cake was washed sequentially with a 90:10 water/methanol mixture (600 mL) and water (900 mL). Finally, it was dried under vacuum to give 195 g (86% yield) of off-white crystalline product.LCMS analysis showed m/z 246 (M-H)+.

3282-30-2
380 suppliers
$19.85/100ml

95-76-1
379 suppliers
$10.00/5g

7160-22-7
65 suppliers
$18.00/250mg
Yield:7160-22-7 195 g (86%)
Reaction Conditions:
with aq NaOH in methanol;water;
Steps:
2 Preparation of N-(3,4-dichlorophenyl)2,2-dimethyl-propionamide.
Preparation of N-(3,4-dichlorophenyl)2,2-dimethyl-propionamide. 3,4-dichloroaniline (150 g) in TBME (1L) was cooled to 10-15° C. 30% aq NaOH (141 g, 1.14 equiv) was added, and the solution stirred vigorously via overhead mechanical stirrer., Trimethylacetyl chloride ("PivCl", 126 mL) was added at such a rate as to keep the internal temperature below 30° C. During this addition, the solution mixture becomes thick with white solid product. When the addition was complete (10-15 min), the mixture was heated to 30-35° C. for 1 hr, and then allowed to cool. The reaction mixture was held at -5° C. (overnight), and then filtered, rinsing first with 90:10 water/MeOH (600 mL) and then water (900 mL). Drying under vacuum yielded 195 g (86%) product, as off-white crystals. LCMS m/z 246(M-H)+.
References:
US2003/216375,2003,A1