
3,3-DIMETHYLCYCLOBUTANECARBOXYLIC ACID synthesis
- Product Name:3,3-DIMETHYLCYCLOBUTANECARBOXYLIC ACID
- CAS Number:34970-18-8
- Molecular formula:C7H12O2
- Molecular Weight:128.17
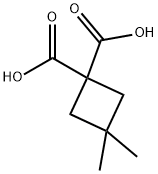
231303-93-8

34970-18-8
The general procedure for the synthesis of 3,3-dimethylcyclobutanecarboxylic acid from the compound (CAS:231303-93-8) was as follows: intermediate 285C (250 mg, 1.452 mmol) was dissolved in pyridine (5 mL) and the reaction was stirred for 16 h at 120 °C. After completion of the reaction, the reaction mixture was cooled to room temperature and the reaction was quenched with 1.5 N HCl aqueous solution. Subsequently, extraction was carried out with ether (2 x 50 mL) at 0 °C. The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford intermediate 285D as a viscous liquid (170 mg, 91% yield).1H NMR (400 MHz, DMSO-d6) δ ppm: 2.90-3.02 (m, 1H), 1.84-1.95 (m, 4H), 1.07-1.14 (m, 3H) , 0.99-1.07 (m, 3H).

231303-93-8
2 suppliers
inquiry

34970-18-8
88 suppliers
$83.00/100 mg
Yield: 91%
Reaction Conditions:
with pyridine at 120; for 16 h;
Steps:
Intermediate 285D: 3,3-Dimethylcyclobutanecarboxylic acid
A solution of Intermediate 285C (250 mg, 1.452 mmol) in pyridine (5 mL) was stirred at 120° C. for 16 h.
The reaction mixture was then cooled to RT, quenched with a 1.5 N aq.
solution of HCl at 0° C., and extracted with diethyl ether (2*50 mL).
The combined organic layers were washed with brine, dried over Na2SO4 and evaporated to afford Intermediate 285D as a viscous liquid (170 mg, 91% yield).
1H NMR (400 MHz, DMSO-d6) δ ppm 2.90-3.02 (m, 1H), 1.84-1.95 (m, 4H), 1.07-1.14 (m, 3H), 0.99-1.07 (m, 3H).
References:
Bristol-Myers Squibb Company;Velaparthi, Upender;Darne, Chetan Padmakar;Liu, Peiying;Wittman, Mark D.;Pearce, Bradley C.;Araujo, Erika M. V.;Dasgupta, Bireshwar;Nair, Jalathi Surendran;Janakiraman, Sakthi Kumaran;Rachamreddy, Chandrasekhar Reddy;Rao, Mettu Mallikarjuna;Karuppiah, Arul Mozhi Selvan Subbiah;Reddy, Bandreddy Subba;Nagalakshmi, Pulicharla;Bora, Rajesh Onkardas;Maheshwarappa, Shilpa Holehatti;Kumaravel, Selvakumar;Mullick, Dibakar;Sistla, Ramesh US9273058, 2016, B2 Location in patent:Page/Page column 329; 330; 331
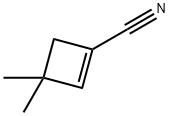
37676-94-1
1 suppliers
inquiry

34970-18-8
88 suppliers
$83.00/100 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

34970-18-8
88 suppliers
$83.00/100 mg
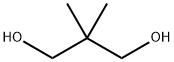
126-30-7
641 suppliers
$8.00/100g

34970-18-8
88 suppliers
$83.00/100 mg
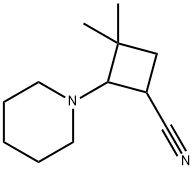
19251-72-0
0 suppliers
inquiry

34970-18-8
88 suppliers
$83.00/100 mg