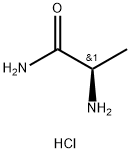
(2R)-2-Aminopropanamide hydrochloride synthesis
- Product Name:(2R)-2-Aminopropanamide hydrochloride
- CAS Number:71810-97-4
- Molecular formula:C3H9ClN2O
- Molecular Weight:124.57
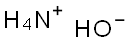
1336-21-6
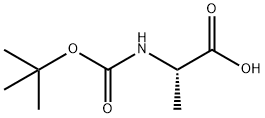
3744-87-4
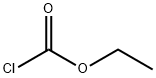
541-41-3

71810-97-4
The general procedure for the synthesis of D-alaninamide hydrochloride from ammonia, (tert-butoxycarbonyl)alanine and ethyl chloroformate is as follows: 1. Boc-D-alanine (5.0 g, 26.43 mmol) was dissolved in anhydrous THF (100 mL). 2. triethylamine (4 mL, 2.91 g, 28.76 mmol) was added to the solution, followed by slow dropwise addition of ethyl chloroformate (2.5 mL, 2.951 g, 27.19 mmol). 3. The reaction mixture was placed in a carbon tetrachloride/dry ice cold bath with continuous stirring for about 30 minutes. 4. Concentrated ammonium hydroxide solution (5 mL, 74.0 mmol) was added, then the reaction mixture was transferred to a refrigerator and allowed to stand overnight. 5. Upon completion of the reaction, the solvent was removed by vacuum evaporation and the residue was dissolved in ethyl acetate. 6. The ethyl acetate layer was washed using saturated sodium carbonate solution and water, followed by drying the organic phase with anhydrous magnesium sulfate. 7. The desiccant was removed by filtration and the solvent was evaporated to obtain the crude product. 8. The crude product was treated with saturated hydrochloric acid/dioxane solution for 1 hour at room temperature. 9. The dioxane was removed by vacuum evaporation and the residue was dissolved in a small amount of methanol and precipitated by the addition of ether to give D-alaninamide hydrochloride (2.5 g, 76% yield).
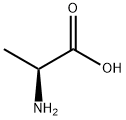
302-72-7
657 suppliers
$8.00/100g
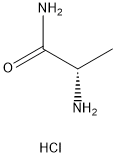
33208-99-0
393 suppliers
$6.00/5g

71810-97-4
197 suppliers
$5.00/1g
Yield:33208-99-0 92%
Reaction Conditions:
with laccase;ammonia in water at 38 - 40;Enzymatic reaction;Temperature;
Steps:
1-3 Example 2
Add 10 grams of DL-alanine and 50 grams of 25% ammonia in a 250 ml single-neck flask, add 60 grams of water for dilution, and stir under the condition of stirring. The temperature of the system rises to 38-40°C, and 0.1 grams of laccase is added. After the reaction is completed for 16-18 hours, the laccase is filtered out and stored in the refrigerator, ready for the next application.The filtrate was directly fed with hydrogen chloride until the pH value of the system reached between 4.3-4.5 to precipitate solids, filtered and dried to obtain 9.2 grams of L-propionamide hydrochloride.The yield is 92% based on the mass of D,L-alanine.
References:
CN112661779, 2021, A Location in patent:Paragraph 0010; 0022-0027
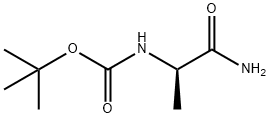
78981-25-6
55 suppliers
$108.00/5 g

71810-97-4
197 suppliers
$5.00/1g