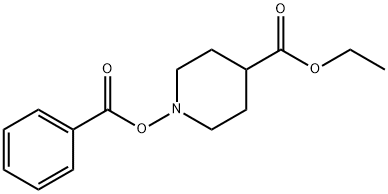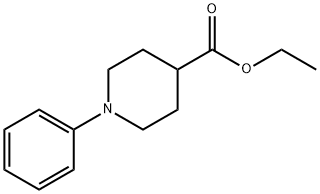
4-Piperidinecarboxylic acid, 1-phenyl-, ethyl ester synthesis
- Product Name:4-Piperidinecarboxylic acid, 1-phenyl-, ethyl ester
- CAS Number:247022-37-3
- Molecular formula:C14H19NO2
- Molecular Weight:233.31
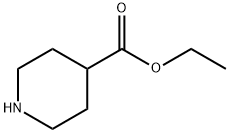
1126-09-6
491 suppliers
$15.40/25g
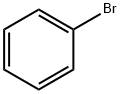
108-86-1
519 suppliers
$10.00/5g

247022-37-3
21 suppliers
inquiry
Yield:247022-37-3 72%
Reaction Conditions:
with 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl;sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0) in toluene at 85; for 1.5 h;
Steps:
293 Reference Example 293; ethyl 1-phenylpiperidine-4-carboxylate
To a solution of tetrakisdibenzylidene acetone dipalladium (PD2 (dba) 3) (173 mg, 0.36 mmol), BINAP (560 mg, 0.9 mmol) and sodium tert-butoxide (1.3 g, 13.5 mmol) in toluene (dry, 20 mL) were added bromobenzene (1.6 g, 10 mmol) and ethyl isonipecotinate (1.7 g, 11 mmol) and the mixture was stirred at 85°C for 1.5 hrs. To the reaction solution was added diethyl ether (20 mL) and insoluble materials were celite filtered. The mother liquor was concentrated. The residue was purified by silica gel column chromatography to give the title compound as a slightly yellow oil (1.7 g, yield 72%). : L H NMR (400 MHz, CDC13) 8 PPM : 1.27 (t, J = 7.1 Hz, 3 H), 1.82 - 1. 93 (m, 2 H), 2. 00-2. 05 (m, 2 H), 2.36-2. 47 (TT, J = 10.9, 3.9 Hz, 1 H), 2.78 (TT, J = 12.1, 2.2 Hz, 2 H), 3. 62- 3. 67 (m, 2 H), 4.16 (q, J = 7.1 HZ, 2 H), 6. 82-6. 95 (m, 3 H), 7.23-7. 28 (m, 2 H). LC/MS (ESI) m/z 234 (M+H+).
References:
WO2004/46107,2004,A1 Location in patent:Page 226-227

1126-09-6
491 suppliers
$15.40/25g

591-50-4
505 suppliers
$10.00/1g

247022-37-3
21 suppliers
inquiry

1126-09-6
491 suppliers
$15.40/25g
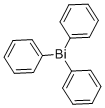
603-33-8
267 suppliers
$30.00/5g

247022-37-3
21 suppliers
inquiry