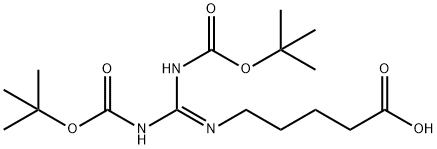
5-(2,3-bis(tert-butoxycarbonyl)guanidino)pentanoic acid synthesis
- Product Name:5-(2,3-bis(tert-butoxycarbonyl)guanidino)pentanoic acid
- CAS Number:212567-95-8
- Molecular formula:C16H29N3O6
- Molecular Weight:359.42
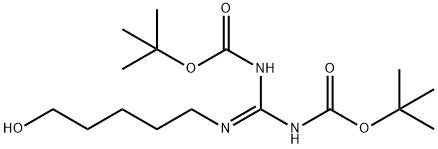
170983-44-5
2 suppliers
inquiry

212567-95-8
21 suppliers
$310.00/100mg
Yield:212567-95-8 176 mg
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;[bis(acetoxy)iodo]benzene in water;acetonitrile at 20; for 3 h;
Steps:
5-((2,2,10,10-Tetramethyl-4,8-dioxo-3,9-dioxa-5,7-diazaundecan-6-ylidene)amino)pentanoic acid (18):
To a solution of 16 (163 mL, 1.50 mmol) in MeOH (7.5 mL) was added N,N’-Bis(tert-butoxycarbonyl)-1H-pyrazole-1-carboxamidine (466 mg, 1.65 mmol) and DIPEA (514 mL, 2.95 mmol) at room temperature. After the mixture was stirred for 2 h at room temperature, the mixture was extracted with EtOAc. The organic layer was dried over MgSO4, then concentrated under reduced pressure to obtain the crude 17. To the crude 17 in CH3CN (4.8 mL)/H2O (4 mL) was added PhI(OAc)2 (1.45 g, 4.50 mmol) and TEMPO (70.3 mg, 0.450 mmol) at room temperature. After the mixture was stirred for 3 h at room temperature, the mixture was extracted with EtOAc. The organic layer was dried over MgSO4, then concentrated under reduced pressure, followed by purification with silica gel column chromatography (n-hexane/EtOAc = 2/1) to provide the title compound 18 (176 mg, 33% (2 steps)) as white solid; 1H-NMR (400 MHz, CDCl3) d 1.50 (s, 9H), 1.51 (s, 9H) 1.65-1.71 (m, 4H), 2.42 (t, J = 7.0 Hz, 2H), 3.47-3.50 (m, 2H), 8.45 (s, 1H), 11.49 (br, 1H); 13C-NMR (100 MHz, CDCl3) d 22.0, 28.2, 28.4, 28.6, 33.5, 40.6, 79.6, 83.3, 153.4, 156.3, 163.5, 178.3; HRMS (ESI), m/z calcd for C16H30FN3O6+ [M+H]+ 360.2129 found 360.2126.
References:
Tsuji, Kohei;Kobayakawa, Takuya;Konno, Kiju;Masuda, Ami;Takahashi, Kohei;Ohashi, Nami;Yoshimura, Kazuhisa;Kuwata, Takeo;Matsushita, Shuzo;Harada, Shigeyoshi;Tamamura, Hirokazu [Bioorganic and Medicinal Chemistry,2022,vol. 56,art. no. 116616] Location in patent:supporting information
![Carbamic acid, N-[[[(1,1-dimethylethoxy)carbonyl]amino]-1H-pyrazol-1-ylmethylene]-, 1,1-dimethylethyl ester, [N(Z)]-](/CAS/20211123/GIF/1143572-00-2.gif)
1143572-00-2
5 suppliers
inquiry
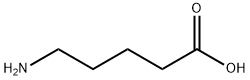
660-88-8
258 suppliers
$6.00/1g

212567-95-8
21 suppliers
$310.00/100mg

2508-29-4
211 suppliers
$11.00/5g

212567-95-8
21 suppliers
$310.00/100mg
![Carbamic acid, N-[[[(1,1-dimethylethoxy)carbonyl]amino]-1H-pyrazol-1-ylmethylene]-, 1,1-dimethylethyl ester, [N(Z)]-](/CAS/20211123/GIF/1143572-00-2.gif)
1143572-00-2
5 suppliers
inquiry

212567-95-8
21 suppliers
$310.00/100mg