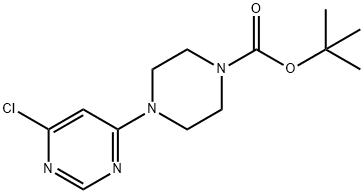
4-(6-Chloro-pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(6-Chloro-pyrimidin-4-yl)-piperazine-1-carboxylic acid tert-butyl ester
- CAS Number:203519-88-4
- Molecular formula:C13H19ClN4O2
- Molecular Weight:298.77
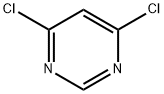
1193-21-1
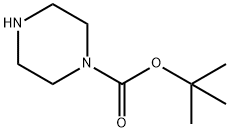
57260-71-6

203519-88-4
Step 1: Synthesis of tert-butyl 4-(6-chloropyrimidin-4-yl)piperazine-1-carboxylate In a microwave reaction tube, 4,6-dichloropyrimidine (0.5 g, 3.36 mmol), N-BOC-piperazine (0.69 g, 3.36 mmol) and triethylamine (0.34 g, 3.36 mmol) were dissolved in acetonitrile (10 mL). The reaction mixture was microwaved at 120 °C for 2 hours. After completion of the reaction, the mixture was diluted with ethyl acetate, washed sequentially with saturated ammonium chloride solution and dried over anhydrous magnesium sulfate. The drier was removed by filtration and the filtrate was concentrated under reduced pressure to afford the target compound tert-butyl 4-(6-chloropyrimidin-4-yl)piperazine-1-carboxylate (0.82 g, 91% yield). 1H NMR (500MHz, CDCl3): δ 8.39 (s, 1H), 6.50 (s, 1H), 3.65 (m, 4H), 3.52 (m, 4H), 1.48 (s, 9H).

1193-21-1
569 suppliers
$13.00/5g
221050-88-0
89 suppliers
$23.00/250mg

203519-88-4
45 suppliers
$265.00/250mg
Yield: 72%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);4,5-bis(diphenylphosphino)-9,9-dimethylxanthene;sodium t-butanolate in toluene at 100;Inert atmosphere;Buchwald-Hartwig Coupling;
Steps:
25 5.1.23 tert-Butyl 4-(2-chloropyridin-4-yl)piperazine-1-carboxylate (29)
General procedure: A mixture of 4-bromo-2-chloropyridine (3.87 mL, 34.9 mmol), N-Boc-piperazine (5.00 g, 26.9 mmol), sodium tert-butoxide (3.87g, 40.3mmol), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene(932.2 mg, 1.61 mmol), tris(dibenzylideneacetone)dipalladium (0) (492mg, 0.537mmol), and toluene (270mL) was stirred at 100°C overnight under N2 atmosphere. The mixture was diluted with water, and extracted with EtOAc. The organic layer was washed with water, dried over anhydrous MgSO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane-EtOAc) to give 29 (5.62g, 70%) as a colorless powder.
References:
Kono, Mitsunori;Matsumoto, Takahiro;Imaeda, Toshihiro;Kawamura, Toru;Fujimoto, Shinji;Kosugi, Yohei;Odani, Tomoyuki;Shimizu, Yuji;Matsui, Hideki;Shimojo, Masato;Kori, Masakuni [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 4,p. 1468 - 1478]

1193-21-1
569 suppliers
$13.00/5g

57260-71-6
734 suppliers
$5.00/5g

203519-88-4
45 suppliers
$265.00/250mg

57260-71-6
734 suppliers
$5.00/5g

203519-88-4
45 suppliers
$265.00/250mg