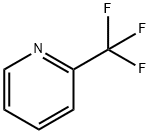
2-(Trifluoromethyl)pyridine synthesis
- Product Name:2-(Trifluoromethyl)pyridine
- CAS Number:368-48-9
- Molecular formula:C6H4F3N
- Molecular Weight:147.1
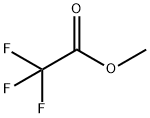
109-04-6
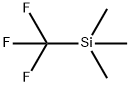
81290-20-2

368-48-9
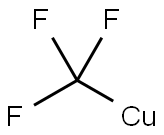
AgF (1.27 g, 10 mmol) was dissolved in 10 mL of DMF at room temperature and stirred thoroughly. Subsequently, Me3SiCF3 (1.7 g, 12 mmol) was added to the mixture and stirring was continued for 20 minutes. Next, copper powder (1.0 g, 15 mmol) was added and the reaction mixture was stirred for 4 hours to ensure complete formation of CuCF3. Then, the corresponding halogenated compound (9 mmol, 4.5 mmol for 2,6-dibromopyridine) was added and the reaction mixture continued to be stirred under the conditions listed in Table 1. The progress of the reaction was monitored by 19F NMR spectroscopy until the signal of CuCF3 completely disappeared and the reaction was terminated. Upon completion of the reaction, the mixture was filtered to remove the solid precipitate and the filtrate was poured into 50 mL of water. The organic layer in the aqueous phase was extracted with ether and the extract was dried with MgSO4. Finally, the ether was removed by evaporation and the residue was purified by distillation under reduced pressure or crystallization to give the target product 2-trifluoromethylpyridine.

109-04-6
539 suppliers
$6.00/25g
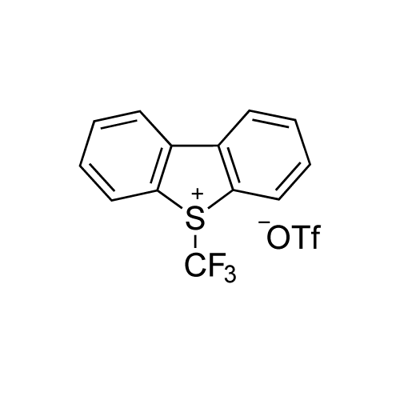
129946-88-9
147 suppliers
$12.00/100mg

368-48-9
224 suppliers
$8.00/1g
Yield:368-48-9 96%
Reaction Conditions:
with copper in N,N-dimethyl-formamide at 0 - 80; for 4 h;Inert atmosphere;
Steps:
6 Example 6: Preparation of 2-trifluoromethylpyridine
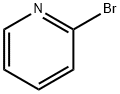
2-bromopyridine (1.58 g, 0.01 mol) was added to a 100 ml three-necked flask under N2 protection.25 ml of DMF, Cu powder (1.92 g, 0.03 mol), stirring was started, and the ice water bath was cooled to 0-5 °C. add Umemoto reagent (8.76g, 0.02mol).After stirring for 1 h in an ice water bath, the mixture was further heated to 80 ° C for 3 h.The reaction solution was subjected to 19F NMR analysis using OTf as an internal standard, and the yield was 96%.
References:
CN108239021,2018,A Location in patent:Paragraph 0060-0062