
2-Pyridineboronic acid synthesis
- Product Name:2-Pyridineboronic acid
- CAS Number:197958-29-5
- Molecular formula:C5H6BNO2
- Molecular Weight:122.92
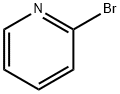
109-04-6
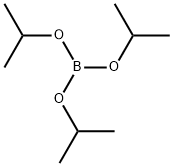
5419-55-6

197958-29-5
The general procedure for the synthesis of 2-pyridineboronic acid from 2-bromopyridine and triisopropyl borate was as follows: 1.58 g (10 mmol) of 2-bromopyridine and 3.76 g (20 mmol) of triisopropyl borate were dissolved in a solvent mixture of 14 mL of anhydrous toluene and 7 mL of THF, stirred and cooled at -30 °C. Under nitrogen protection, 6.25 mL (10 mmol) of 1.6 M n-butyllithium hexane solution was added slowly dropwise with a syringe. After the dropwise addition, the reaction mixture was continued to be stirred at -30 °C for 3 h. Subsequently, it was brought to room temperature and stirred for another 3 h. The completion of the reaction was monitored by TLC. 1 mL of concentrated hydrochloric acid was slowly added to the reaction mixture and stirred for 1 hr at room temperature before the mixture was poured into 200 mL of ice water. The pH was adjusted to 8 with saturated sodium bicarbonate solution, stirred, and extracted three times with 50 mL of dichloromethane. The organic phases were combined, washed with 100 mL of 5% brine and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the filtrate was concentrated to dryness by rotary evaporator, and the residue was purified by silica gel column chromatography to give 1.00 g of 2-pyridineboronic acid in 81% yield.

109-04-6
539 suppliers
$6.00/25g

5419-55-6
392 suppliers
$12.00/5g

197958-29-5
205 suppliers
$12.00/250mg
Yield:197958-29-5 81%
Reaction Conditions:
Stage #1:2-bromo-pyridine;Triisopropyl borate with n-butyllithium in tetrahydrofuran;hexane;toluene at -30; for 3 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;hexane;water;toluene at 20; for 1 h;Inert atmosphere;
Steps:
1.2 Step 2. Synthesis of Compound VI-1
1.58 g (10 mmol) of compound V-1 and 3.76 g (20 mmol) of B(i-PrO)3 were dissolved in 14 mL of dry toluene and 7 mL of THF, stirred and cooled to -30°C under a nitrogen atmosphere, and then slowly dried with a syringe. A solution of 6.25 mL (10 mmol) of 1.6 M n-BuLi in n-hexane was added dropwise. After completion of the addition, the reaction mixture was stirred at this temperature for 3 hours and then at room temperature for a further 3 hours, and the reaction was completed by TLC. To the reaction mixture was slowly added 1 mL of concentrated hydrochloric acid, stirred at room temperature for 1 hour, and poured into 200 mL of ice water.Where, pH=8 was adjusted with saturated NaHCO 3 solution, stirred, extracted with 50 mL×3 CH 2 Cl 2 , the combined extracts were washed with 100 mL 5% brine and dried over anhydrous sodium sulfate. The desiccant was filtered off with suction, the filtrate was evaporated to dryness on a rotary evaporator and the residue was purified using silica gel column chromatography to afford compound VI-I, 1.00 g (81% yield).
References:
Foshan Sai Weisi Pharmaceutical Technology Co., Ltd.;Cai Ziyang CN107903250, 2018, A Location in patent:Paragraph 0025; 0029; 0030; 0031

109-04-6
539 suppliers
$6.00/25g
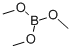
121-43-7
385 suppliers
$13.00/25mL

197958-29-5
205 suppliers
$12.00/250mg

109-04-6
539 suppliers
$6.00/25g

197958-29-5
205 suppliers
$12.00/250mg