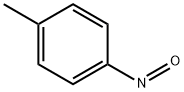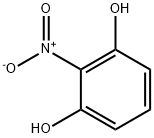
2-Nitroresorcinol synthesis
- Product Name:2-Nitroresorcinol
- CAS Number:601-89-8
- Molecular formula:C6H5NO4
- Molecular Weight:155.11
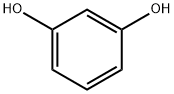
108-46-3
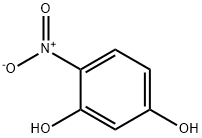
3163-07-3

601-89-8
At room temperature, 1 mmol of resorcinol was dissolved in 40 mL of phosphate buffer solution of pH=7, followed by the addition of 60 mmol of sodium nitrite. 4 mmol of hydrogen peroxide was dissolved in 10 mL of phosphate buffer solution and added to the reaction system after 15 min. Subsequently, 0.06 g of Fe-Al-MCM-41 molecular sieves was added to initiate the reaction, and the reaction was stirred for 80 min at room temperature. After completion of the reaction, the filtrate was extracted with ethyl acetate (2 x 80 mL), and the organic phases were combined and concentrated under reduced pressure. The target products 2-nitroresorcinol (27.4% yield) and 4-nitroresorcinol (23.5% yield) were isolated by column chromatography purification (silica gel column, eluent was a mixture of petroleum ether and acetone, 1:1, v/v/v/v/v), and the total yield was 50.9%. Under the same reaction conditions, when 0.06 g of water-soluble tetrakis(sodium sulfonate) iron porphyrin was used as the catalyst instead of molecular sieves, the yields of 2-nitroresorcinol were 9.9%, 4-nitroresorcinol were 11.8%, and the total yield was 21.7%.
Yield: 27.4% , 23.5%
Reaction Conditions:
with dihydrogen peroxide;sodium nitrite in aq. phosphate buffer; pH=7 at 20; for 1.58 h;Green chemistry;Reagent/catalyst;Concentration;
Steps:
9
At room temperature, 1 mmol of resorcinol was dissolved in 40 mL of phosphate buffer at pH = 7,Then, 60 mmol of sodium nitrite was added and 4 mmol of hydrogen peroxide was dissolved in 10 mL of phosphate buffer,in15 min into the reaction solution, at room temperature by adding 0.06g Fe-Al-MCM-41 molecular sieve to start the reaction, stirring reaction 80min. The filtrate was extracted with ethyl acetate (2 x 80 mL), concentrated under reduced pressure,Column chromatography (column packed with silica gel, eluted with petroleum ether and acetone as eluent,Petroleum ether and acetone volume ratio of 1: 1, that is, the target product 2 - nitroresorcinol, the yield of 27.4%And 4 - nitroresorcinol, the yield was 23.5% and the total yield was 50.9%. Under the same conditions,In the absence of the use of molecular sieve but 0.06gThe reaction was initiated using a water-soluble tetrakis (sodium sulfonate-based) iron porphyrin.As a result, the yield of 2-nitroresorcin was 9.9%, the yield of 4-nitroresorcin was 11.8%, The total yield was 21.7%.
References:
Qingdao Institute of Biomass Energy and Bioprocess Technology, Chinese Academy of Sciences;SUN, Wei-zhi;XU, Chao;XIAN, Mo;LIU, Fusheng;LIU, Yao-jie CN106045803, 2016, A Location in patent:Paragraph 0045-0046

108-46-3
795 suppliers
$10.00/10g

601-89-8
241 suppliers
$14.00/5g

576-26-1
345 suppliers
$10.00/25g

601-89-8
241 suppliers
$14.00/5g
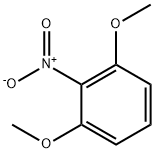
6665-97-0
60 suppliers
$60.00/100mg

107-06-2
440 suppliers
$14.00/25g

601-89-8
241 suppliers
$14.00/5g