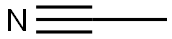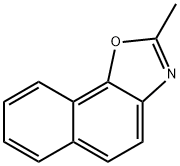
2-METHYLNAPHTHO(2,1-D)OXAZOLE synthesis
- Product Name:2-METHYLNAPHTHO(2,1-D)OXAZOLE
- CAS Number:20686-65-1
- Molecular formula:C12H9NO
- Molecular Weight:183.21
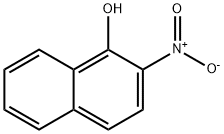
607-24-9
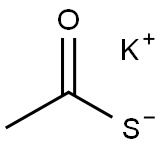
10387-40-3

20686-65-1
General procedure for the synthesis of 2-methylnaphtho[2,1-D]oxazoles from 2-nitro-1-naphthol and potassium thioacetate: A surfactant-mediated solvent-free scheme was used, which can also be applied to the conversion of aryl nitro compounds to aryl acetamides. This is achieved by reacting a mixture of aryl nitro compounds (1 eq.) with potassium thioacetate (4 eq.) in the presence of dry Triton-X 405 (as catalyst) at ca. 130 °C for ca. 3 hours in a solvent-free acetamidation reaction. The reaction produces the corresponding aryl acetamides with higher than 95% conversion (by HPLC and GC analysis) and selectivity. Representative results for the acetamidation of aryl nitro compounds have been summarized in Table 2. The generalized reaction formula for the acetamidation of aryl nitro compounds is shown below.

20937-86-4
5 suppliers
inquiry

20686-65-1
75 suppliers
$10.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: polyphosphoric acid / 140 - 145 °C
2: 80 °C / 0.1 Torr
References:
Mullock,E.B.;Suschitzky,H. [Journal of the Chemical Society C: Organic,1968,p. 1937 - 1940]
![Acetamide, N-[1-(acetyloxy)-2-naphthalenyl]-](/CAS/20210305/GIF/857945-08-5.gif)
857945-08-5
0 suppliers
inquiry

20686-65-1
75 suppliers
$10.00/250mg

607-24-9
168 suppliers
$9.00/1g

10387-40-3
358 suppliers
$10.00/1g
70365-38-7
0 suppliers
inquiry

20686-65-1
75 suppliers
$10.00/250mg

20937-86-4
5 suppliers
inquiry
64-19-7
1603 suppliers
$10.00/25ML

20686-65-1
75 suppliers
$10.00/250mg