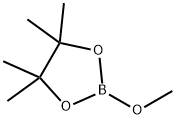
2-Methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-Methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:1195-66-0
- Molecular formula:C7H15BO3
- Molecular Weight:158
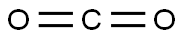
124-38-9
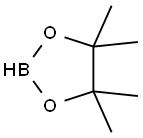
25015-63-8
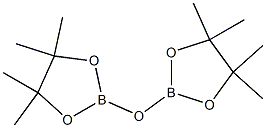
10221-56-4

1195-66-0
The general procedure for the synthesis of 2,2'-oxybis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) and 2-methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane from carbon dioxide and pinacolborane was as follows: a solution of (PhPSiP)Ni(η3-C8H13) (1.0 mg, 0.0014 mmol) and 1 ,3,5-trimethoxybenzene (11.5 mg, 0.068 mmol) were added to a J.YY NMR tube and the solution was frozen. After cooling, a layer of pinacolborane (9.9 μL, 0.068 mmol) was added. On a double Schlenk line, the headspace was evacuated and passed through 1 atm of CO2 at room temperature.After 15 min, almost complete depletion of pinacolborane was observed, along with the appearance of three major products. The products were identified by comparison with those reported by Sabo-Etienne.After 15 min, products a, b, and c were present in a 61:5:34 ratio with unreacted pinacolborane. However, the product c was gradually consumed over time, and after 12 h, all pinacolborane was consumed, leaving only traces of product c in the reaction mixture.The product integrals showed the presence of 13% 2-methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborole pentacyclic, 85% 2,2'-oxybis(4,4,5,5-tetramethyl-1,3,2-dioxaborole heterocyclopentane) and a small amount of unidentified by-products. In addition, a peak corresponding to formaldehyde at 8.72 ppm was observed.
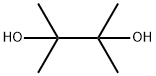
76-09-5
396 suppliers
$8.19/250mg
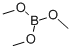
121-43-7
385 suppliers
$13.00/25mL

1195-66-0
189 suppliers
$5.00/1g
Yield:1195-66-0 90%
Reaction Conditions:
for 6 h;Inert atmosphere;Reflux;
Steps:
Synthesis of 2-methoxy-4,4,5,5-tetramethyl[1,3,2]dioxaborolane
Synthesis of 2-methoxy-4,4,5,5-tetramethyl[1,3,2]dioxaborolane A flask equipped with a reflux condenser, stirrer, an inert gas inlet and a thermocouple set in heating mantle was charged with trimethyl borate and pinacol under positive pressure of nitrogen. The resulting mixture was heated at reflux for 6 hours. The mixture was cooled to ambient temperatures and transferred to a flask with a Vigreux column. The mixture was distilled at atmospheric pressure to afford 2-methoxy-4,4,5,5-tetramethyl[1,3,2]dioxaborolane (bp 159° C.) in high yield (90%). 1H NMR (400 MHz, CDCl3): δ 3.41 (m, 3H), 1.11 (m, 12H). 11B NMR (128.3 MHz, CDCl3): δ 22.2. 13C NMR (100.6 MHz, CDCl3): δ 82.7, 52.6, 24.6.
References:
Anderson Development Company US2012/289733, 2012, A1 Location in patent:Page/Page column 4
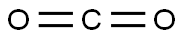
124-38-9
132 suppliers
$175.00/23402

25015-63-8
223 suppliers
$22.39/5G

10221-56-4
2 suppliers
inquiry

1195-66-0
189 suppliers
$5.00/1g
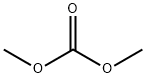
616-38-6
719 suppliers
$5.00/5 g

25015-63-8
223 suppliers
$22.39/5G

1195-66-0
189 suppliers
$5.00/1g
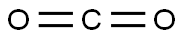
124-38-9
132 suppliers
$175.00/23402

25015-63-8
223 suppliers
$22.39/5G

1195-66-0
189 suppliers
$5.00/1g

67-56-1
781 suppliers
$7.29/5ml-f
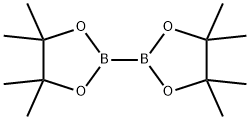
73183-34-3
587 suppliers
$6.00/5g

1195-66-0
189 suppliers
$5.00/1g

1333-74-0
116 suppliers
$190.00/56L