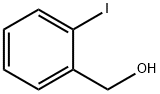
2-Iodobenzyl alcohol synthesis
- Product Name:2-Iodobenzyl alcohol
- CAS Number:5159-41-1
- Molecular formula:C7H7IO
- Molecular Weight:234.03

88-67-5

5159-41-1
The general procedure for the synthesis of 2-iodobenzyl alcohol from 2-iodobenzoic acid was as follows: to a tetrahydrofuran (THF) solution (16 mL) of commercially available o-iodobenzoic acid (4.15 g, 16.8 mmol) was slowly added borane-dimethyl sulfide complex (1.90 mL, 20.0 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 15 hours before the reaction was quenched by the addition of phosphate buffer (pH 7). Subsequently, the reaction mixture was extracted three times with ethyl acetate and the organic phases were combined. The organic phase was washed with saturated brine and dried with anhydrous sodium sulfate. Concentration under reduced pressure to remove the solvent gave o-iodobenzyl alcohol (3.84 g, 98% yield) as a white solid, which could be used in the next reaction without further purification. The crude o-iodobenzyl alcohol (3.77 g, 16.1 mmol) was added to a dichloromethane (DCM, 33 mL) suspension of dimethyl sulfoxide (DMSO, 14.0 g, 161 mmol). The reaction mixture was stirred at room temperature for 30 h before being filtered through a diatomaceous earth pad, using dichloromethane as the eluent. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 5:1) to afford o-iodobenzaldehyde (3.36 g, 90% yield) as light yellow crystals.

610-97-9
423 suppliers
$9.00/1g

5159-41-1
222 suppliers
$8.00/5g
Yield:-
Reaction Conditions:
with sodium tetrahydroborate;[fac-8-(2-diphenylphosphinoethyl)amidotrihydroquinoline]RuH(PPh3)(CO);hydrogen in tetrahydrofuran at 120; under 38002.6 Torr; for 12 h;Autoclave;Industrial scale;
Steps:
3-5 example 3-5
General procedure: (1 mmol) of NaBH44, 3.08 g (20 mmol, S: B: C: 2000: 20: 1) of methyl o-fluorobenzoate were sequentially added to 100 mL of a glass bottle in a ratio of 7.51 mg ),The reaction system was placed in a 100mL autoclave, replaced with hydrogen three times, rushed into hydrogen 50atm, temperature set at 120 , heating and stirring reaction 18 hours, the reactor was placed in an ice bath to room temperature, venting gas .GC analysis of the reaction solution specific results in Table III. In addition to o-iodobenzoate 5.24g (20mol, S: B: C = 2000: 20: 1), heated and stirred 12 hours, itshydrogenation reduction reaction performed with the same as in Example 3-1, the specific results in Table three.
References:
Hebei Normal University;Liu, Qingbin;Wang, Zheng;Liu, Bo;Zhang, Fujun CN105237342, 2016, A Location in patent:Paragraph 0023; 0027; 0040

88-67-5
565 suppliers
$5.00/5g

5159-41-1
222 suppliers
$8.00/5g
26260-02-6
245 suppliers
$8.00/250mg

5159-41-1
222 suppliers
$8.00/5g
18698-96-9
251 suppliers
$5.00/250mg

5159-41-1
222 suppliers
$8.00/5g

80953-51-1
1 suppliers
inquiry

5159-41-1
222 suppliers
$8.00/5g