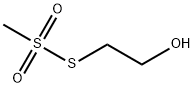
2-Hydroxyethyl Methanethiosulfonate synthesis
- Product Name:2-Hydroxyethyl Methanethiosulfonate
- CAS Number:13700-08-8
- Molecular formula:C3H8O3S2
- Molecular Weight:156.22

1950-85-2
74 suppliers
$9.00/250mg

540-51-2
258 suppliers
$17.00/5g

13700-08-8
18 suppliers
$150.00/10mg
Yield:-
Reaction Conditions:
in ethanol; for 7 h;Heating / reflux;
Steps:
4
EXAMPLE 4; Synthesis of (Z) -5-Fluoro-2-methyl-1- [ [4- (methylsulfinyl) phenyl] methylene] -IH-indene-3 -acetic acid 2-methanesulfonylsulfanylethyl ester; Sodium sulphide nonahydrate (4 g; 16.32 mmol) is EPO
References:
WO2006/134489,2006,A1 Location in patent:Page/Page column 15-17
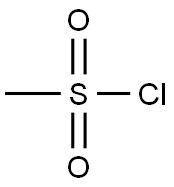
124-63-0
0 suppliers
$10.00/5g

540-51-2
258 suppliers
$17.00/5g

13700-08-8
18 suppliers
$150.00/10mg