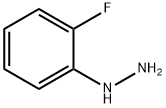
2-Fluorophenylhydrazine synthesis
- Product Name:2-Fluorophenylhydrazine
- CAS Number:2368-80-1
- Molecular formula:C6H7FN2
- Molecular Weight:126.13
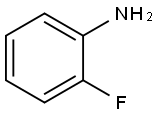
348-54-9

2368-80-1
General procedure for the synthesis of 2-fluorophenylhydrazine from o-fluoroaniline: 500 g of o-fluoroaniline and 1500 mL of 37% concentrated hydrochloric acid were added to a 10 L three-necked flask and cooled to 2 °C using an ice-salt bath. 857 g of 35% aqueous sodium nitrite solution was added slowly and dropwise under stirring, and the reaction temperature was controlled to be maintained at 0 to 5 °C for 1.5 hours. Subsequently, 730 mL of 37% concentrated hydrochloric acid, 730 mL of water, and 480 g of zinc powder were added to the reaction solution, and the reaction temperature was maintained at 18 °C until the reaction was complete and the reaction solution turned off-white. Then, 30% sodium hydroxide solution was slowly added to adjust the pH of the reaction solution to 10.5, and this process took about 2 hours, and then crystals were precipitated, and 295 g of 2-fluorophenylhydrazine crude product was obtained by filtration. Purification step: 295 g of 2-fluorophenylhydrazine crude was dissolved in 5900 mL of water, heated to 60 ℃ to make it completely dissolved, and added appropriate amount of activated carbon to decolorize for 20 minutes, and then thermo-filtered to obtain a colorless filtrate. The filtrate was allowed to stand for 5 hours and then continued to stand for 2 hours, and the precipitated crystal precipitate was collected by filtration to finally obtain 238g of purified 2-fluorophenylhydrazine.
Yield: 74%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;sodium hydrogen sulfate;sodium nitrite
Steps:
4 Preparation of 2-fluorophenylhydrazine with addition of n-butanol
Example 4 Preparation of 2-fluorophenylhydrazine with addition of n-butanol In a 2 l four-necked flask fitted with dropping funnel, stirrer and condenser, 240 g of HCl 30% strength and 180 g of n-butanol (saturated with water, approximately 80% strength) are initially charged. The solution is heated to 50° C. and, over a period of 25 minutes, admixed with a total of 1291 g of an aqueous solution of 2-fluorophenylhydrazinedisulfonate--prepared by diazotization of 111 g (1 mol) of 2-fluoroaniline (156 g of water, 303 g of HCl 30% strength, 170 g of NaNO2) and subsequent reduction with sulfite (560 g of NaHSO3 +100 g of NaOH). The reaction solution is kept at 50° C. for another 60 minutes. During the reaction, the reaction system forms two phases. The two phases are separated from one another in a 2 l separating funnel. The aqueous phase is subsequently extracted three times with 10 ml of n-butanol each time. The organic phases are combined and adjusted to pH 8.5 using aqueous sodium hydroxide solution (33% strength), and the aqueous phase that forms is also separated off. The solvent is subsequently concentrated until a viscous crystal slurry is formed. Yield: 98.2 g (0.74 mol) of 2-fluorophenylhydrazine (95% pure), this corresponds to a theoretical yield of 74.0%, based on the 2-fluoroaniline employed.
References:
Clariant GmbH US6057478, 2000, A

348-54-9
431 suppliers
$10.00/1g

2368-80-1
158 suppliers
inquiry
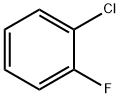
348-51-6
221 suppliers
$21.00/5 g

2368-80-1
158 suppliers
inquiry
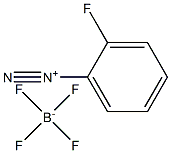
446-46-8
1 suppliers
inquiry

2368-80-1
158 suppliers
inquiry
