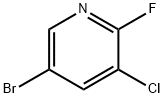
5-Bromo-3-chloro-2-fluoropyridine synthesis
- Product Name:5-Bromo-3-chloro-2-fluoropyridine
- CAS Number:38185-56-7
- Molecular formula:C5H2BrClFN
- Molecular Weight:210.43

38185-55-6

38185-56-7
2) The reaction system was cooled in an ice bath, followed by the slow addition of sodium nitrite (4.8 g, 70 mmol) to a solution consisting of the above amine product (12.6 g, 61 mmol) dissolved in hydrogen fluoride/pyridine (70% HF, 91 ml). The reaction mixture was stirred continuously for 30 min at room temperature. After completion of the reaction, ice was added to the reaction solution and neutralized with sodium carbonate solution. Subsequently, the organic layer was separated by extraction with ether. The organic layer was washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate and finally concentrated under reduced pressure to remove the solvent. The crude product obtained was further purified by silica gel column chromatography (eluent ratio hexane:ethyl acetate=80:20) to afford the target compound 5-bromo-3-chloro-2-fluoropyridine (11.1 g, 87% yield) as a white solid.

38185-55-6
149 suppliers
$7.00/100mg

38185-56-7
135 suppliers
$9.00/1g
Yield: 87%
Reaction Conditions:
with pyridine;hydrogen fluoride;sodium nitrite at 20;Cooling with ice;
Steps:
20.2 Preparation of 3-chloro-5-[2-(1,3-dioxolan-2-yl)ethoxy]-2-fluoropyridine
2) With cooling with ice, sodium nitrite (4.8 g, 70 mmol) was added to a hydrogen fluoride/pyridine (70 % HF) solution (91 ml) of the amine product (12.6 g, 61 mmol) obtained in the above reaction, and stirred at room temperature for 30 minutes. Ice was added to the reaction solution, neutralized with sodium carbonate, extracted with diethyl ether. The organic layer was washed with water and saturated saline water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (hexane:ethyl acetate = 80:20) to obtain 5-bromo-3-chloro-2-fluoropyridine (11.1 g, yield: 87 %) as a white solid.
References:
Banyu Pharmaceutical Co., Ltd. EP2221301, 2010, A1 Location in patent:Page/Page column 46