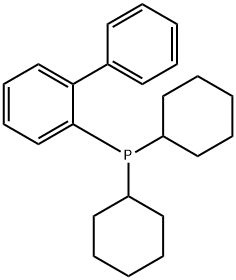
2-(Dicyclohexylphosphino)biphenyl synthesis
- Product Name:2-(Dicyclohexylphosphino)biphenyl
- CAS Number:247940-06-3
- Molecular formula:C24H31P
- Molecular Weight:350.48

2052-07-5
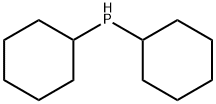
829-84-5

247940-06-3
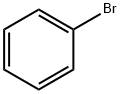
General procedure for the synthesis of 2-(dicyclohexylphosphino)biphenyl from 2-bromobiphenyl and dicyclohexylphosphine: under nitrogen protection, a mixture of L1 (0.05 or 0.10 mmol P), [PdCl(η3-C3H5)]2 (0.025 mmol), 2-bromobiphenyl (0.55 mmol) and dicyclohexylphosphine (0.50 mmol) was reacted in a 20 M KOH The mixture in aqueous solution (0.5-1.0 mL) was reacted by shaking at 100 °C for several hours. After completion of the reaction, the mixture was cooled to room temperature and filtered. The aqueous filtrate was extracted with degassed toluene (2 mL x 2 times). For the recovered catalyst resin beads, extract with degassed toluene (2 mL x 4 times). All extracts were combined and dried with anhydrous Na2SO4, followed by concentration under vacuum to obtain the crude product. The crude product was purified by silica gel fast chromatography using degassed eluent (n-hexane/Et2O = 10/0-9/1), and the purification process was completed by a YAMAZEN medium-pressure liquid chromatography system, which ultimately yielded the target product 2-(dicyclohexylphosphino)biphenyl.

2052-07-5
390 suppliers
$16.30/1g

829-84-5
154 suppliers
$24.19/1g

247940-06-3
284 suppliers
$6.00/1g
Yield:247940-06-3 81%
Reaction Conditions:
with bis(η3-allyl-μ-chloropalladium(II));potassium hydroxide in water at 100;Inert atmosphere;
Steps:
General procedure for the C-P cross coupling
General procedure: A mixture of L1 (0.05 or 0.10 mmol of P), [PdCl(η3-C3H5)]2 (0.025mmol), arylhalides (0.55 mmol) and dicyclohexylphosphine (0.50 mmol) in 20 M KOH aqueous solution (0.5-1.0 mL) under nitrogen atmosphere was shaken for several hours at 100 °C. The mixture was cooled and filtered. After being cooled, the reaction mixture was filtered and the aqueous filtrate was extracted with degassed toluene (2 mL x 2 times). Recovered catalyst resin beads were extracted with degassed toluene (2 mL x 4 times). The combined extract was dried over anhydrous Na2SO4 and concentrated in vacuo to give a crude residue. The residue was purified by silica-gel flash chromatography to give an aryl dicyclohexylphosphine 3. Chromatographic purification was carried out with the YAMAZEN medium pressure liquid chromatograph system using degassed eluent (n-hexane/Et2O = 10/0-9/1).
References:
Hirai, Yoshinori;Uozumi, Yasuhiro [Synlett,2017,vol. 28,# 20,p. 2966 - 2970] Location in patent:supporting information

2052-07-5
390 suppliers
$16.30/1g
16523-54-9
273 suppliers
$8.00/1g

247940-06-3
284 suppliers
$6.00/1g
108-86-1
525 suppliers
$10.00/5g
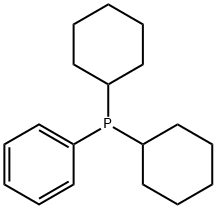
6476-37-5
168 suppliers
$30.00/250mg

247940-06-3
284 suppliers
$6.00/1g
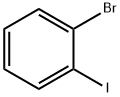
583-55-1
481 suppliers
$7.00/5g

247940-06-3
284 suppliers
$6.00/1g
![Methanesulfonic acid, 1,1,1-trifluoro-, [1,1'-biphenyl]-2-yl ester](/CAS/20200611/GIF/17763-65-4.gif)