
2-chloroquinolin-8-ol synthesis
- Product Name:2-chloroquinolin-8-ol
- CAS Number:31568-91-9
- Molecular formula:C9H6ClNO
- Molecular Weight:179.6
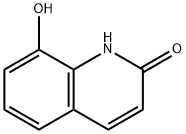
15450-76-7

31568-91-9
General procedure for the synthesis of 2-chloro-8-hydroxyquinoline from 2,8-quinolinediol: Thionyl chloride (3.56 ml, 49.6 mmol) was added slowly and dropwise to a suspension of N,N-dimethylformamide (20 ml) in 2,8-quinolinediol (2.00 g, 12.4 mmol) under ice-bath cooling and stirring conditions. The reaction mixture was stirred at room temperature for 2 h. After that, thionyl chloride (3.56 ml, 49.6 mmol) was added again and the reaction temperature was raised to 50 °C and stirring was continued for 3.5 h. The reaction mixture was then stirred for 3.5 h. The reaction temperature was raised to 50 °C and stirring was continued. After completion of the reaction, the reaction solution was cooled to room temperature, poured into ice water and extracted with ethyl acetate. The organic phase was washed with water, dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane=1/20) to afford 2-chloro-8-hydroxyquinoline (1.72 g, 77% yield) as a pale yellow solid. The product had a melting point of 79-80 °C, APCI-MS m/z 180/182 [M+H]+.

15450-76-7
303 suppliers
$6.00/1g

31568-91-9
69 suppliers
$57.50/250 mg
Yield:31568-91-9 77%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide at 20 - 50; for 5.5 h;Cooling with ice;
Steps:
1 Synthesis of 50
Synthesis of 50
To a N,N-dimethylformamide (20 ml) suspension of 1 (2.00 g, 12.4 mmol), thionyl chloride (3.56 ml, 49.6 mmol) was added dropwise under ice cooling and stirring, and the mixture was stirred at room temperature for 2 hours, and then thionyl chloride (3.56 ml, 49.6 mmol) was added, followed by further stirring at 50°C for 3.5 hours. The reaction solution was allowed to return to room temperature, poured into ice water and extracted with ethyl acetate. The extraction liquid was washed with water and dried, and then the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (eluting solvent: ethyl acetate/n-hexane = 1/20) to obtain 50 (1.72 g, 77%) as a pale yellow solid. mp 79-80°C, APCI-MS m/z 180/182[M+H]+
References:
Clino Ltd.;KUDO, Yukitsuka;FURUMOTO, Syozo;OKAMURA, Nobuyuki EP2634177, 2013, A1 Location in patent:Paragraph 0217; 0218
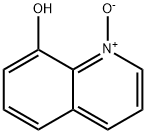
1127-45-3
235 suppliers
$6.00/1g

31568-91-9
69 suppliers
$57.50/250 mg
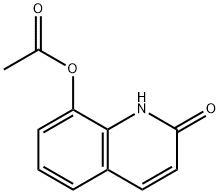
15450-72-3
44 suppliers
inquiry

31568-91-9
69 suppliers
$57.50/250 mg

15450-72-3
44 suppliers
inquiry

31568-91-9
69 suppliers
$57.50/250 mg
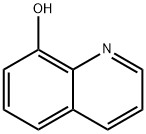
148-24-3
867 suppliers
$5.00/25g

31568-91-9
69 suppliers
$57.50/250 mg