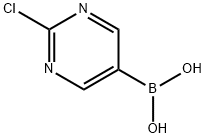
2-CHLOROPYRIMIDINE-5-BORONIC ACID synthesis
- Product Name:2-CHLOROPYRIMIDINE-5-BORONIC ACID
- CAS Number:1003845-06-4
- Molecular formula:C4H4BClN2O2
- Molecular Weight:158.35
5419-55-6

32779-36-5

1003845-06-4
Step 1: Synthesis of 2-chloropyrimidin-5-ylboronic acid Under argon protection, 5-bromo-2-chloropyrimidine (1.0 g, 5.170 mmol) was dissolved in a solvent mixture of THF and toluene (25 mL, 4:1 v/v), and cooled to -78 °C. n-Butyllithium (1.6 M hexane solution, 3.87 mL, 5.61 mmol) was slowly added dropwise at -78 °C, and the reaction was kept at -78 °C for 4 hours after completion of the dropwise addition. After completion of the reaction, the reaction mixture was diluted with ice water, returned to room temperature and stirred for 1 hour. Subsequently, the aqueous phase was extracted with ether and the organic phase was combined. The aqueous phase was acidified to pH 2-3 with 1N hydrochloric acid and extracted with ethyl acetate. All organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give 2-chloropyrimidin-5-ylboronic acid (0.6 g, 73.3% yield) as a white solid. Mass spectrum (MS): 159.3 [M+H]+.

5419-55-6
392 suppliers
$12.00/5g

32779-36-5
649 suppliers
$5.00/5g

1003845-06-4
196 suppliers
$18.69/250mg
Yield:1003845-06-4 73.3%
Reaction Conditions:
with hydrogenchloride;n-butyllithium in tetrahydrofuran;hexane;toluene at -78; for 4 h;
Steps:
25.1 Step 1: Synthesis of 2-chloropyrimidin-5-yl-5-boronic acid
Step 1:
Synthesis of 2-chloropyrimidin-5-yl-5-boronic acid
To a stirred solution of 5-bromo-2-chloropyrimidine (1.0 g, 5.170 mmol) in mixture of THF:Toluene (25 mL, 4:1) was added n-BuLi (1.6M in Hexane) (3.87 mL, 5.61 mmol) dropwise at -78° C.
And allowed to stir at -78° C. for 4 h.
On completion, Reaction mass diluted with water and stirred at RT for 1 h, extracted with Diethyl ether.
Then acidify using 1N HCl up to pH 2-3and extracted with EtOAc.
Organic portions were combined, dried over Na2SO4, evaporated under reduced pressure to give 2-chloropyrimidin-5-yl-5-boronic acid (0.6 g, 73.3%) as white solid.
MS: 159.3[M++1]
References:
Mankind Pharma Ltd.;Patil, Rakesh Ishwar;Verma, Jeevan;Shah, Dharmesh;Ali, Sazid;Bapuram, Srinivasa Reddy;Rai, Santosh Kumar;Kumar, Anil US2017/291910, 2017, A1 Location in patent:Paragraph 0508-0510

32779-36-5
649 suppliers
$5.00/5g

1003845-06-4
196 suppliers
$18.69/250mg