
2-CHLOROPYRIMIDINE-4-CARBOXAMIDE synthesis
- Product Name:2-CHLOROPYRIMIDINE-4-CARBOXAMIDE
- CAS Number:22536-66-9
- Molecular formula:C5H4ClN3O
- Molecular Weight:157.56

149849-92-3

22536-66-9
General procedure for the synthesis of 2-chloropyrimidine-4-carboxamide from 2-chloropyrimidine-4-carboxylic acid: 2-chloropyrimidine-4-carboxylic acid (500 mg, 1.00 eq.) was dissolved in thionyl chloride (10 mL) and the reaction was heated to 90 °C for 30 min. Upon completion of the reaction, the excess thionyl chloride was removed by concentration under reduced pressure. The residue was cooled to 0 °C and ammonium hydroxide solution (20 mL) was slowly added. The reaction mixture was extracted with dichloromethane (20 mL x 3), the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the organic phase was concentrated under reduced pressure to give 320 mg of 2-chloropyrimidine-4-carboxamide as a white solid. The product was analyzed by LC-MS (electrospray ionization, m/z): 158 [M+H]+.

149849-92-3
195 suppliers
$6.00/250mg

22536-66-9
94 suppliers
$30.00/100mg
Yield:22536-66-9 92%
Reaction Conditions:
Stage #1: 2‐chloropyrimidine‐4‐carboxylic acidwith thionyl chloride in toluene; for 10 h;Reflux;
Stage #2: with ammonium hydroxide at 20; for 1 h;Cooling with ice;
Steps:
3 Example 3
A 100 mL three-necked reaction flask was added with 50 mL of thionyl chloride, and 2-chloro-pyrimidine-4-carboxylic acid (2c) (15.9 g, 100 mmol) was added in batches under stirring conditions, and the addition was completed in 10 minutes. After the addition, the reaction mixture was refluxed and reacted for 10 hours, the thionyl chloride was distilled off, and 10 mL of N,N-dimethylformamide was added to the residue to dissolve. In addition, 150 mL of ammonia water (25-28% content) was added to a 250 mL three-necked reaction flask, cooled in an ice water bath, and the above acid chloride solution was dripped at constant pressure, and reacted at room temperature for 1 hour after dripping. After the reaction, it was filtered, the solid was washed with 3×20 mL of water, and dried to obtain the solid product 2-chloro-pyrimidine-4-carboxamide (3c) (14.5 g, yield 92%). The target product was confirmed by nuclear magnetic resonance spectroscopy.
References:
CN112851677,2021,A Location in patent:Paragraph 0030-0033
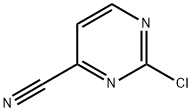
75833-38-4
174 suppliers
$27.00/1g

22536-66-9
94 suppliers
$30.00/100mg
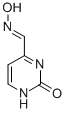
7460-56-2
35 suppliers
$45.00/50mg

22536-66-9
94 suppliers
$30.00/100mg
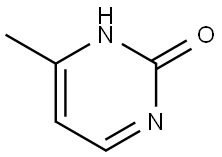
15231-48-8
107 suppliers
$24.00/250mg

22536-66-9
94 suppliers
$30.00/100mg