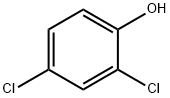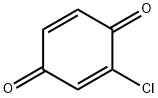
2-CHLORO-1,4-BENZOQUINONE synthesis
- Product Name:2-CHLORO-1,4-BENZOQUINONE
- CAS Number:695-99-8
- Molecular formula:C6H3ClO2
- Molecular Weight:142.54
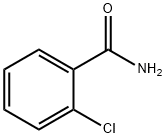
609-66-5

695-99-8
GENERAL METHOD: Diacetoxyiodobenzene (5 mmol, 1.61 g) and NaHSO4-H2O (1 mmol, 0.138 g) were stirred in an aqueous acetonitrile solution (5 mL of water and 5 mL of acetonitrile) for 10-15 minutes at room temperature. To this reaction mixture, 2-chlorobenzamide (1 mmol) was added and stirring was continued until the reaction was complete (monitored by TLC). Upon completion of the reaction, the reaction mixture was quenched with ice water and subsequently extracted with chloroform (3 x 10 mL). The chloroform layers were combined, washed with water (3 x 20 mL), dried with anhydrous Na2SO4 and concentrated on a rotary evaporator to give the crude product. The crude product was further purified by silica gel column chromatography using ethyl acetate: hexane (1:9, v/v) as eluent to give pure 2-chloro-1,4-benzoquinone.
Yield: 25% , 5%
Reaction Conditions:
with dihydrogen peroxide;Ti-superoxide in water;acetic acid at 60 - 70; for 1.25 h;Product distribution / selectivity;
Steps:
14
Preparation of 2-chloro-1, 4-benzoquinone A mixture of 2, 4-dichlorophenol (5 mmol) and Ti-superoxide catalyst (125 mg, 20% w/w) in acetic acid (5 ml) was heated with stirring at 60-70 C under inert atmosphere. To this reaction mixture was added aq. 30% H202 (20 mmol) drop wise over 15 min. and heated for 1 h. The catalyst was recovered by simple filtration and corresponding quinone formed (25%) was separated by chromatographic purification.Table 1: Ti-superoxide (1) catalyzed oxidation of phenols to quinines and hydroquinones with aq. 30% H202 : a Ex. Substrate t/h Conversion ProductU Selectivity (%) c No. (%) Quinone HQd 1. Phenol 10 22 2 20e 91. 0 2. Phenol 8 66 5 6 92. 4 3. Phenol 7 65 3 60 92.3 4. Phenol 7 68 3.5 63g 92.7 5. Phenol 1 92 88 2.3 95.7 6. o-Cresol 1 99 96 3.0 97.0 7. 7n-Cresol 1 100 99 0.5 99.0 8. 2,6-Dimethylphenol 1 100 97 2.0 97.0 9. 2-'Butylphenol 1 99 97 0. 7 98.0 10. 2, 6-Dibutylphenol 3 70 65 2. 0 93.0 11.4-Chlorophenol 1 57 55-96.5 12.4-Bromophenol 1 61 60-98. 4 13. 4-Iodophenol 1 77 75-97.4 14.2, 4-Dichlorophenol 1 32 25 5. 0 78.1 (a) See examples for detailed experimental procedure; (b) determined by GC analysis of the crude product; (c) selectivity to hydroquinone ; (d) hydroquinone; (e) aq. 10% H202; (f) aq.50% H202; (g) 40% w/w catalyst.
References:
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH WO2005/63664, 2005, A1 Location in patent:Page/Page column 8; Table 1

609-66-5
270 suppliers
$10.00/5g

695-99-8
89 suppliers
$40.00/1g
615-67-8
188 suppliers
$13.00/5g

695-99-8
89 suppliers
$40.00/1g
![Benzene, 2-chloro-1,4-bis[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/67289-08-1.gif)
67289-08-1
0 suppliers
inquiry

695-99-8
89 suppliers
$40.00/1g
108-90-7
682 suppliers
$10.00/25G

695-99-8
89 suppliers
$40.00/1g