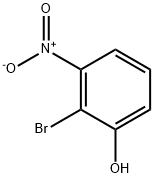
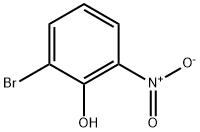
2-Bromo-3-nitrophenol synthesis
- Product Name:2-Bromo-3-nitrophenol
- CAS Number:101935-40-4
- Molecular formula:C6H4BrNO3
- Molecular Weight:218

603-85-0

101935-40-4
General procedure for the synthesis of 2-bromo-3-nitrophenol from 2-amino-3-nitrophenol: 2-amino-3-nitrophenol (5 g, 32.4 mmol) was dissolved in water (29.5 mL) and 1,4-dioxane (14.7 mL). The mixture was heated to reflux and hydrobromic acid (48%, 16.7 mL, 147 mmol) was added dropwise over 20 min. After addition, reflux was continued for 15 min. The reaction mixture was cooled to 0 °C (ice bath) and a solution of sodium nitrite (2.23 g, 32.3 mmol) in water (20 mL) was added over 30 min. Stirring was continued at 0 °C for 15 min, then the mixture was transferred to a pre-cooled jacketed dropping funnel (0 °C) and added dropwise to a stirred mixture of cuprous bromide (I) (5.34 g, 37.2 mmol) in water (29.5 mL) and hydrobromic acid (48%, 16.7 mL, 147 mmol). The reaction mixture was stirred at 0 °C for 15 min, then warmed to 60 °C, continued stirring for 15 min, cooled to room temperature, and stirred overnight. The reaction mixture was transferred to a partition funnel and extracted with ether (3 x 150 mL). The organic layers were combined, washed with brine (1 x 150 mL), dried (Na2SO3), filtered and concentrated to give the crude product (7.99 g) as a reddish brown oil. The crude product was extracted by fast column chromatography (1:25 ultrapure silica gel, 230-400 mesh, 40-60 mm, 60 ? ; CH2Cl2 as solvent) purified the crude product to afford pure 2-bromo-3-nitrophenol (45%, 3.16 g) as an orange-brown solid. Mass spectrum (MS) m/z: 217.8 (MH+). High performance liquid chromatography (HPLC, TFA) homogeneity at 220 nm: 97%.
67853-37-6
138 suppliers
$8.00/1g

101935-40-4
135 suppliers
$9.00/100mg
Yield: 98%
Reaction Conditions:
with boron tribromide in dichloromethane at -70 - 20; for 26 h;Product distribution / selectivity;Inert atmosphere;
Steps:
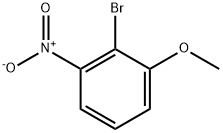
SSynthesis of 2-f2'-Nitro-6'-pyridin-4-yl-biphenyl-4-yloxymethyl)-αuinoline (Example 384)2-Bromo-3-nitrophenol Error. Objects cannot be created from editing field codes.BBr3 (1.0M in CH2Cl2, 88 mL, 88 mmol) was added dropwise over 1 h to a stirred solution of 2-bromo-3-nitroanisole in CH2Cl2 (35 mL) under argon at -70 0C. The resulting deep burgundy-colored reaction mixture was allowed to warm up to RT slowly (over 2 h) and stirred at RT for 23 h. The reaction mixture was poured onto 35O g crushed ice and extracted with EtOAc (300 mL). The organic phase was separated, washed with brine (75 mL), and dried over MgSO4. Concentration and purification by chromatography (5-70% EtO Ac/heptane) gave the title compound 2-bromo-3-nitrophenol (5.36 g, 98%) as a yellow solid. 1H NMR (300 MHz, CDCI3/TMS) δ 7.48 (d, J= 8.1 Hz, IH), 7.37 (t, J = 8.1 Hz, IH), 7.27 (d, J= 8.4 Hz, IH), 6.13 (br s, IH); 13C NMR (75 MHz, CDCI3/TMS) δ 153.7, 128.7, 119.8, 117.5, 102.9.
References:
ENVIVO PHARMACEUTICALS, INC. WO2009/158467, 2009, A2 Location in patent:Page/Page column 59-60; 91

603-85-0
272 suppliers
$15.00/5g

101935-40-4
135 suppliers
$9.00/100mg

5344-78-5
239 suppliers
$6.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

101935-40-4
135 suppliers
$9.00/100mg
7486-35-3
275 suppliers
$15.00/1g
112970-61-3
1 suppliers
inquiry

101935-40-4
135 suppliers
$9.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
13073-25-1
340 suppliers
$6.00/1g

101935-40-4
135 suppliers
$9.00/100mg
